Abstract
Aims
To explore short echo time (30 ms) 1 H magnetic resonance spectroscopy (MRS) in children with brain tumours and determine the contributions to the characterization of these tumours of the metabolites inositol/myoinositol and glutamate/glutamine, which are not visible at long echo times (135 or 270 ms).
Methods
Over a 12-month period 86 single-voxel MRS investigations were performed on 59 children with various brain tumours on a Siemens Symphony 1.5-T Magnetom using point-resolved spectroscopy and echo time of 30 ms.
Results
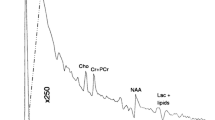
The procedure was well tolerated, and good-quality data were obtained. N-Acetyl aspartate (NAA)/Choline (Cho) and creatine (Cr)/Cho concentration ratios were significantly (p<0.001) lower in tumour (0.95 and 1.63, respectively) compared with non-involved brain (3.68 and 3.98, respectively) in all histological types. Inositol/Myoinositol (Inos)/Cho ratios were significantly (p<0.05) lower in untreated tumours (1.91) than in treated tumours (3.93) and in non-involved brain (3.32). Inos/Cho ratios were high in diffuse pontine gliomas and low in medulloblastomas and supratentorial primitive neuroectodermal tumours (p<0.01). Glutamate/Glutamine (Glut)/Cho ratios were high in grade 1 astrocytomas (6.4) and unbiopsied optic gliomas (9.84) but low in diffuse pontine gliomas (2.44). Lipids and macromolecules were present in most tumours but in low quantities in non-involved brain.
Conclusion
Good-quality short echo time MRS data can be collected routinely on children with brain tumours. Inos and Glut levels show greater variability between tumour types than NAA, Cho and Cr present at long echo times, providing improved tumour characterization. Inos/Cho levels differ between untreated and treated tumours and may be useful for treatment monitoring.
Similar content being viewed by others
References
Astrakas LG, Zurakowski D, Tzikka AA et al (2004) Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin Cancer Res 10:8220–8228
Burtscher IM, Skagerberg G, Geijer B et al (2000) Proton MR spectroscopy and preoperative diagnostic accuracy: an evaluation of intracranial mass lesions characterised by stereotactic biopsy findings. Am J Neuroradiol 21:84–93
Girard N, Wang ZJ, Erbetta A et al (1998) Prognostic value of proton MR spectroscopy of cerebral hemisphere tumours in children. Neuroradiology 40:121–125
Howe FA, Opstad KS (2003) 1 H MR spectroscopy of brain tumours and masses. NMR Biomed 16:123–131
Lindskog M, Kogner P, Ponthan F et al (2003) Non invasive estimation of tumour viability in a xenograft model of human neuroblastoma with proton magnetic resonance spectroscopy (1 H MRS). Br J Cancer 88:478–485
Muckherji SK (ed) (1998) Clinical applications of MR spectroscopy. Wiley-Liss, New York
Opstad KS, Provencher SW, Bell BA et al (2003) Detection of elevated glutathione in meningiomas by quantitative in vivo 1 H MRS. Magn Reson Med 49:632–637
Peet A, Garala P, MacPherson L, Natarajan K, Sgouros S, Grundy R (2004) Mobile lipids detected by short echo time 1 H magnetic resonance spectroscopy correlate with malignancy in childhood brain tumours. Neuro-oncol 6:471
Peet A, Garala P, MacPherson L, Natarajan K, Sgouros S, Grundy R (2004) Short echo time 1 H magnetic resonance spectroscopy of childhood brain tumours. Childs Nerv Syst 20(5):256
Peet AC, Leach MO, Pinkerton CR, Price P, Williams SR, Grundy RG (2005) The development of functional imaging for the diagnosis, management and understanding of childhood brain tumours—an EPSRC workshop. Paediatr Blood Cancer 44:103–113
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Pruel MC, Caramanos Z, Collins DL et al (1996) Accurate, noninvasive diagnosis of human brain tumours by using proton magnetic resonance spectroscopy. Nat Med 2:323–325
Pruel MC, Caramanos Z, Leblanc R et al (1998) Using pattern analysis of in vivo proton MRSI data to improve the diagnosis and surgical management of patients with brain tumours. NMR Biomed 11:192–200
Thiesse P, Jaspan T, Couanet D et al (2001) Un Protocols d’imager des tumeurs cerebrales de l’enfant (A protocol for imaging paediatric brain tumours). J Radiol 82:177–191
Tzika AA, Astrakas LG, Zarifi MK et al (2003) Multiparametric MR assessment of paediatric brain tumours. Neuroradiology 2002:1–10
Tzika AA, Astrakas LG, Zarifi MK et al (2004) Spectroscopic and perfusion magnetic resonance imaging predictors of progression in pediatric brain tumours. Cancer 100:1246–1256
Tzika AA, Zarifi MK, Goumnerova L et al (2002) Neuroimaging in pediatric brain tumours: Gd-DTPA-enhanced hemodynamic, and diffusion MR imaging compared with MR spectroscopic imaging. Am J Neuroradiol 23:322–333
Vaidya SJ, Payne GS, Leach MO et al (2003) Potential role of magnetic resonance spectroscopy in assessment of tumour response in childhood cancer. Eur J Cancer 39:728–735
Wilke M, Eidenschink A, Muller-Weihrich S, Auer DP (2001) MR diffusion imaging and 1 H spectroscopy in a child with medulloblastoma. A case report. Acta Radiol 42:39–42
Wilkinson ID, Griffiths PD, Wales JK (2001) Proton magnetic resonance spectroscopy of brain lesions in children with neurofibromatosis type 1. Magn Reson Imag 19:1081–1089
Acknowledgements
Some of the materials of this paper were presented during the XIX Biennial Congress of the European Society for Pediatric Neurosurgery in conjunction with the Japanese Society for Pediatric Neurosurgery and the Korean Society for Pediatric Neurosurgery, Rome, Italy, 6 May 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peet, A.C., Lateef, S., MacPherson, L. et al. Short echo time 1 H magnetic resonance spectroscopy of childhood brain tumours. Childs Nerv Syst 23, 163–169 (2007). https://doi.org/10.1007/s00381-006-0206-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-006-0206-4