Abstract
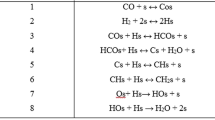
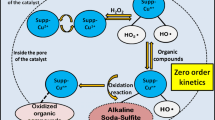
In this paper, we analyse the model for the UV-C photo-Fenton degradation of acetic acid using \(LaFeO_{3}\) heterogeneous structured catalyst with a monolithic structure. The model is based on the occurrence of hydrogen peroxide photolysis and acetic acid oxidation in the heterogeneous phase, assumed first-order and Eley–Rideal type kinetics respectively. The main objective is to propose an analytical solution for the concentrations of \(\hbox {CH}_{3}\hbox {COOH}\) and \(\hbox {H}_{2}\hbox {O}_{2}\). A powerful analytical method called Homotopy Perturbation Method (HPM) has been employed to obtain approximate analytical solutions. The accuracy of the analytical solution is tested with the numerical solution. In this work the numerical solution of the problem is reported using SCILAB program. Stability Analysis of this model is also explained. The obtained analytical solution in comparison with the numerical and stability analysis is found to be in satisfactory agreement.




Similar content being viewed by others
References
D. Krzeminska, E. Neczaj, G. Borowski, Advanced oxidation processes for food industrial wastewater decontamination. J. Ecol. Eng. 16(2), 61–71 (2015)
T.L.P. Dantas, H.J. Jose, R.F.P.M. Moreira, Fenton and photo-Fenton oxidation of tannery wastewater. Acta Sci. Technol. 25(95), 91–95 (2003)
N. These, Development of a Photo-Fenton Catalyst Supported on Modified Polymer Films. Advanced Oxidation Processes and UV–Visible Spectrum Measurements, Preparation, Characterization and Implication for Water Decontamination by Solar Photocatalysis (2010)
J.Y. Park, In Hwa Lee, eecomposition of acetic acid by advanced oxidation processes. Korean J. Chem. Eng. 26(2), 387–391 (2009)
M. Blanco, A. Martinez, A. Marcaide, E. Aranzabe, A. Aranzabe, Heterogeneous Fenton catalyst for the efficient removal of Azo dyes in water. Am. J. Anal. Chem. 5, 490–499 (2014)
J. Herney-Ramirez, A. Vicente Miguel, M. Madeira Luis, Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: a review. Appl. Catal. B Environ. 98, 10–26 (2010)
F. Ijpelaar Guus, J.H. Harmsen Danny, Minne Heringa, UV Disinfection and UV/ \(\text{ H }_{2}\text{ O }_{2}\) Oxidation: by -product Formation and Control: UV/ \(\text{ H }_{2}\text{ O }_{2}\) Oxidation (2007)
A.S. Dos Santos Fajardo, Treatment of Liquid Effluents by Catalytic Ozonation and Photo Fenton’s Processes: Waterwater’s Treatment by AOPs at Ambient Conditions (2011)
M. Punzi, B. Mattiasson, M. Jonstrup, Treatment of synthetic textile wastewater by homogeneous and heterogeneous photo-Fenton oxidation. J. Photochem. Photobiol. A 248, 30–35 (2012)
I. Kano, D. Darbouret, Stephane Mabic. Photo-Oxidation of Organics Using Dual-Wavelengh UV Lamps, UV Technologies in Water Purification Systems (2003)
H. Van der Vegt, Ilian Iliev. UV Water Treatment, Patent Landscape Report on Membrane Filtration and UV Water Treatment (2012)
Q. Chen, W. Pingxiao, Z. Dang, N. Zhu, P. Li, W. Jinhua, X. Wang, Iron pillared vermiculite as a heterogeneous photo-Fenton catalyst for photocatalytic degradation of Azo dye reactive brilliant orange X-GN. Sep. Purif. Technol. 71, 315–323 (2010)
D. Sannino, V. Vaiano, P. Ciambelli, L.A. Isupova, Mathematical modeling of the heterogeneous photo-Fenton oxidation of acetic acid on structured catalysts. Chem. Eng. J. 224, 53–58 (2013)
O.O. Dosumu, O.O. Oluwaniyi, G.V. Awolola, M.O. Okunola, Stability studies and mineral concentration of some nigerian packed fruit juices, concentrate and local beverages. Adv. Nat. Appl. Sci. 3(1), 22–26 (2009)
M.-L. Tomescu, S. Preitl, R.-E. Precup, K.T. Jozsef, Stability analysis method for fuzzy control systems dedicated controlling nonlinear processes. Acta Polytechnica Hungarica 4(3), 127–141 (2007)
H. Li, X. Bai, X.-S. Yang, Stability results on a chemical system. J. Math. Chem. 43(1), 365–374 (2008)
K. Mallory, A. Van Gorder Robert, A transformed time-dependent Michaelis–Menten enzymatic reaction model and its asymptotic stability. J. Math. Chem. 52, 222–230 (2014)
J.H. He, Homotopy perturbation technique. Comput. Methods Appl. Mech. Eng. 178(3–4), 257–262 (1999)
J.H. He, A coupling method of a homotopy technique and a perturbation technique for non-linear problems. Int. J. Non Linear Mech. 35(1), 37–43 (2000)
J.H. He, Homotopy perturbation method for solving boundary value problems. Phys. Lett. A 350(1–2), 87–88 (2006). doi:10.1016/j.physleta.2005.10.005
S. Loghambal, L. Rajendran, Mathematical modeling of diffusion and kinetics of amperometric immobilized enzyme electrodes. Electrochim. Acta 55, 5230–5238 (2010)
S. Loghambal, L. Rajendran, Mathematical modeling in amperometric oxidase enzyme-membrane electrodes. J. Membr. Sci. 373(1), 20–28 (2011)
P.R.V. Margret, S. Loghambal, L. Rajendran, Approximate solution of coupled system of transient reaction diffusion equations for complexes of arbitrary lability. Global J. Pure Appl. Math. 3(5), 405–426 (2011)
Acknowledgments
The Authors thank the management and the Principal of V V College of Engineering, Tisaiyanvilai for their constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
1.1 Basic idea of homotopy perturbation method
Consider the following non-linear differential equation
with the boundary conditions of
where A, B, f(r), and \(\Gamma \) are a general differential operator, a boundary operator, a known analytic function and the boundary of the domain \(\Omega \), respectively.
The operator A can generally be divided into a linear part L and a nonlinear part N. Equation (18) may therefore be written as,
By the homotopy technique, we construct a homotopy \(v (r,p):\Omega \times [0, 1]\rightarrow R\)which satisfies
or
where \(p\in [0, 1]\) is an embedding parameter, while \(y_0\) is an initial approximation of (18), which satisfies the boundary conditions. Obviously, from (21) and (22) we will have
The changing process of p from zero to unity is just that of v (r, p) from \(y_0\) to y(r). In topology, this is called deformation, while \(L(v)-L(y_0 )\) and \(A(v)-f(r)\) are called homotopy. If the embedding parameter p is considered as a small parameter, applying the classical perturbation technique, we can assume that the solution of (21) and (22) can be written as a power series in p:
Setting \(p= 1\) in (25), we have
The combination of the perturbation method and the homotopy method is called the HPM, which eliminates the drawbacks of the traditional perturbation methods while keeping all its advantages. The series (26) is convergent for most cases. However, the convergent rate depends on the nonlinear operator A(v). Moreover, J.H. He made the following suggestions.
-
1.
The second derivative of N(v)with respect to v must be small because the parameter may be relatively large, that is, \(p\rightarrow 1\).
-
2.
The norm of \(L^{-1}\left( {\frac{\partial N}{\partial v}} \right) \) must be smaller than one so that the series converges.
Appendix 2
Using homotopy perturbation method, we construct a homotopy for the Eqs. (12) and (13) as follows
and
The approximate solution of (27) is as follows:
The approximate solution of (28) is as follows:
Substituting Eqs. (29) and (30) into Eq. (27) and arranging the coefficients of p powers, we have
Substituting Eqs. (29) and (30) into Eq. (28) and arranging the coefficients of p powers, we have
The initial approximations are as follows:
and
and
From Eq. (31) we get
From Eq. (33) we get
Substituting Eqs. (39) and (40) in Eq. (32) we obtain the solution of Eq. (32),
Using Eqs. (39) and (40) in Eq. (34) and then solving we get,
Adding (39) and (41) we get \(f_1 \), similarly adding (40) and (42) we get, \(g_1 \).
Using Eq. (43) we get Eq. (16) and Eq. (17) in the text.
Appendix 3
function catalysts
options= odeset(’RelTol’,1e-6,’Stats’,’on’);
Xo = [1; 0];
tspan = [0,5];
tic
[t,X] = ode45(@TestFunction,tspan,Xo,options);
toc
figure
hold on
plot(t, X(:,1),’-’)
plot(t, X(:,2),’-’)
return
function [dx_dt]= TestFunction(t,x)
y1=0.05;
y2=50;
k=1;
dx_dt(1) =-y1*k*x(1)*x(2)/(1+y1*x(1));
dx_dt(2) =-4*y1*k*x(1)*x(2)/(1+y1*x(1))-x(2)+y2;
dx_dt = dx_dt’;
Rights and permissions
About this article
Cite this article
Loghambal, S., Agvinos Catherine, A.J. Mathematical analysis of the heterogeneous photo-Fenton oxidation of acetic acid on structured catalysts. J Math Chem 54, 1146–1158 (2016). https://doi.org/10.1007/s10910-016-0613-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-016-0613-z