Abstract
Varroa-sensitive hygiene (VSH) is highly influenced by the worker bee’s olfactory ability. Workers bred for VSH and non-selected control line workers were tested for differences in their speed and perception ability when presented with highly diluted stimuli. Four different substances (citral – dilution 1:1300, linalool dilution 1:1300, Varroa-parasitized brood extract, isopropanol) were used as tactile stimuli for differential conditioning with the proboscis extension response (PER). Discrimination ability and generalization were assessed. In a second set of conditioning experiments differences in sensitivity to the highly diluted citral and the Varroa-parasitized brood extract as reinforced stimuli (Cs +) were explored between workers from both lines. The worker bees were classified into three groups (Time points) depending on how long before they started correctly extending their proboscis to the Cs + , and results were examined separately for each of the two stimuli and group. While the VSH-selected line exhibited a significantly higher perception ability for the parasitized brood extract than the non-selected line, the two lines showed no differences when conditioned with the floral stimulus citral as Cs + . Furthermore, the VSH-selected line displayed a significantly higher number of worker bees that perceived the complex bouquet of the Varroa-parasitized brood extract at the earliest time grouping (Time point 1). The odds of perception at the earliest possible time point were 2.6-times higher for the VSH-selected line. Although no comparison was made between healthy and parasitized brood, the results indicate an enhanced specific sensitivity in VSH-selected workers towards chemical cues emitted by the brood, which might play a role in the detection of Varroa destructor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European honey bee, Apis mellifera, is one of the most important agricultural pollinators worldwide. However, since the parasitic mite Varroa destructor shifted hosts from the Asian honey bee Apis cerana to the European honey bee, a global increase in colony loses has been observed for the latter species (Genersch et al. 2010; Dietemann et al. 2012; Martin et al. 2012). While some populations appear to be Varroa-resistant (Locke 2016; Oddie et al. 2017, 2018), most of the honey bee colonies are still dependent on the Varroa-treatment administered by beekeepers (Jacques et al. 2017). However, recent breeding efforts to create bees with enhanced Varroa-sensitive hygiene (VSH) — a specialized type of hygienic behavior comprising the targeting and removal of Varroa-infested brood — have improved bee colonies’ survival in the face of parasitization (Mondet et al. 2020).
Varroa destructor induces a shift in the cuticular hydrocarbon profile of parasitized brood (Nazzi et al. 2004; Wagoner et al. 2019; Mondet et al. 2021) which is detected through the cell cap by nursing bees. Compounds such as tricosan-2-one, pentacosan-2-one, tetracosyl acetate, heptacosan-2-one, hexacosyl acetate and nonacosan-2-one have been detected in extracts of parasitized pupae (Mondet et al. 2021). Furthermore, (Z)-pentadec-6-ene and (Z)-10-tritiacontene, the non-volatile oleic acid, as well as the increase of brood ester pheromone are also able to elicit a hygienic response (Nazzi et al. 2004; Mondet et al. 2016; Wagoner et al. 2020) and are associated with Varroa-parasitization (Wagoner et al. 2021). This odor change acts as a signal for the worker bees and a trigger for VSH (Harbo and Harris 2005; Wagoner et al. 2018). Subsequently, the brood cells are uncapped and the diseased pupae removed (Martin et al. 2002; Swanson et al. 2009). Mondet et al. (2021) observed that while all worker bees can perceive the compounds typical for a V. destructor parasitization at the level of the antennae, only those bees performing VSH can differentiate between these compounds and the odor of unparasitized healthy brood. Moreover, worker bees from colonies bred for VSH are more likely to uncap infested cells with more than one foundress mite (Kim et al. 2018) and brood severely affected by transmitted viruses (Schöning et al. 2012).
The early detection of parasitized brood and the subsequent removal of the mites has been identified as being significantly genetically influenced (Spötter et al. 2012, 2016; Guarna et al. 2015). The differential expression of genes for the olfactory and sensory activity determines the perception ability and olfactory sensitivity of the single worker bee (Boutin et al. 2015; Hu et al. 2016; Gempe et al. 2016). Under laboratory conditions, olfactory ability can be tested with the help of differential conditioning using the proboscis extension response (PER). First described by Takeda in 1961, this method lies at the center of assessing olfactory discrimination abilities in bees (Takeda 1961; Bitterman et al. 1983; Giurfa and Malun 2004; Giurfa 2008; Matsumoto et al. 2012; Smith and Burden 2014). Through a series of trials, a bee learns to differentiate between two odors: Cs + (reinforced with a reward) and Cs- (unreinforced, or novel odor). In order to feed on the reward sugar solution, the bee displays a behavioral change by extending its mouthparts, or proboscis.
PER conditioning can provide valuable information on the differences in perception ability towards various chemicals in lines bred for enhanced hygienic behavior including VSH and non-selected lines. Masterman et al. (2000) observed significantly better discrimination ability in hygienic bees when exposed to the odor of healthy and chalkbrood infested brood compared to non-hygienic bees. Flower odors, on the other hand, were perceived equally well by both groups of bees. Compared to a chalkbrood infection where the brood dies, the parasitization with V. destructor causes amongst others immunosuppression without killing the brood (Rosenkranz et al. 2010; Vidal-Naquet 2015). While chalkbrood mummies elicit a strong stimulus leading to their removal, the changes in the brood during a V. destructor parasitization are likely more subtle, therefore more difficult to sense. A study conducted by Chakroborty et al. (2015) using PER conditioning tested VSH-selected and non-selected worker bees with the odor of healthy and Varroa-parasitized pupae. The study did not deliver conclusive results whether VSH colonies are endowed with better odor discrimination abilities than the non-hygienic colonies. During the experiment, the colonies bred for enhanced hygienic behavior towards V. destructor exhibited only small differences in odor discrimination ability towards the Varroa-infested brood compared the non-hygienic worker bees. These observations on odor sensitivity may be accounted for by the small numbers of tested individuals (N hygienic = 54, N control = 42). The method of presentation (olfactometer) might also play an important factor, considering that some of the compounds extracted from Varroa-parasitized brood are non-volatile and therefore cannot be presented through an air stream (Nazzi et al. 2004).
Here we describe a complementary study aimed at observing the perception ability of worker bees to different stimuli by using learning as a marker for sensitivity. While the PER response does not measure the sensitivity of an individual bee, but the learning behavior to a stimulus, if the stimulus is not detected during a tactile or volatile presentation, there is no learning success even with large differences in learning ability. We therefore defined higher sensitivity as a faster and generally higher perception of the presented stimulus. Bienefeld et al. (2015) displayed that learning does not play a role in hygienic behavior. Rather, hygienic behavior is an instinctive reaction to abnormal cues, with olfactory sensitivity playing a central role (Schöning et al. 2012; Mondet et al. 2015, 2021; Wagoner et al. 2021). We hypothesized that conditioning using the PER can be utilized as a method for quantifying olfactory sensitivity for the use in Varroa-resistance breeding (Ivanova and Bienefeld 2021). Workers from two origins (a VSH-selected line and a non-selected line) were presented with two highly diluted extracts — citral (essential oil, well known for its use in conditioning experiments (Vareschi 1971; Nagaraja and Bruckner 2013) as well as a minor component of the Nasonov pheromone (Shearer and Boch 1966)) and an extract of Varroa-parasitized brood. In order to better define the differences in the perception ability of each group, we used a larger sample size and a lower concentration of Varroa-parasitized brood extract rather than live parasitized pupae as used in the experiments of Chakroborty et al. (2015). A tactile presentation of the extract was chosen as means of delivering the stimuli. We further hypothesized that the undiluted odors used in Chakroborty et al.’s (2015) experiments pose an easy task for the test subjects and provide information on overall odor perception ability but give no feedback on olfactory sensitivity.
The following questions formed the basis for the performed experiments: Is there a difference in the perception ability of worker bees bred for VSH and the non-selected line worker bees, when presented with highly diluted stimuli? Does the perception speed between the two lines differ? Are the differences in the perception ability a result of an overall higher olfactory sensitivity or a specific sensitivity towards cues which are likely to cause VSH?
Materials and Methods
Colonies
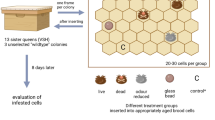
Worker bees from a total of sixteen colonies participated in two PER-conditioning experiments. The colonies were situated at one of the Institute for Bee Research Hohen Neuendorf’s own locations in Brandenburg, Germany (coordinates: 52.66943; 13.39455). Each colony was used only once. Half of the colonies originated from the institute’s VSH-selection program (Bienefeld et al. 2001), while the other half was not selected for VSH but shared a similar genetic background.
The institute’s VSH-selection program comprises video-observation of recognition and uncapping of Varroa-parasitized brood through individual workers in a standardized observation unit using a sample of 40–50 worker bees/mother (Bienefeld et al. 2015). The main selection criterion for the mother queens and father colonies (sperm donors) is the relative proportion of worker bee offspring that has started uncapping Varroa-parasitized brood during a 6-day video-observation. Details on the development of this line will be available in a separate publication.
During the preparations of the conditioning experiments, 50 workers from each colony were gathered as they emerged from the brood cells and marked with numbered plates on the dorsal thorax. Subsequently, they were fostered in a hive with a virgin queen until they were tested. A brood frame with sealed brood was placed in the hive to stimulate nursing behavior. The marked worker bees were tested at an age spanning from 3 to 11 days, with an average age of 6 days, as this time range corresponds with glandular development of the hypopharyngeal glands and nursing behavior as stated by Page and Peng (2001).
Extract Preparation
The Varroa-parasitized brood extract was created as presented by Ivanova and Bienefeld (2021). Twenty newly capped prepupae (9–10 days old) were artificially infested with four Varroa-mites each. Since the distress signal emitted by the brood, rather than the number of mites in the brood cell, is perceived by the nursing bees, we wanted to produce an extract that mimicked the changes in the brood’s cuticular hydrocarbon profile (Bauer et al. 2018; Mondet et al. 2021). By using four mites per prepupa, we ensured that even if mites were damaged during artificial infestation, a sufficiently strong stress factor for the brood would still be present.
The cell caps were cut open on one side and the mites inserted with the help of a moistened brush. The cell caps were subsequently closed. Afterwards, the brood frame was introduced to the hive it came from for two hours, in order for the incisions in the cell caps to be completely resealed by workers (Ivanova and Bienefeld 2021). The brood frame was incubated for four days in an incubator at 35 °C. Subsequently, fifteen parasitized pupae were extracted from the brood cells without being damaged and were soaked in 4 ml isopropanol for 10 min. The supernatant was decanted in 2 ml glass vials with PVC lids (Ivanova and Bienefeld 2021). Between the conditioning experiments, the extract was stored at -20 °C. Five microliters of the extract contained 0.02 brood equivalents.
Both floral stimuli – citral and linalool – were diluted in isopropanol. One microliter of the floral compound was combined with 1299 µl isopropanol using a micropipette. The extracts were stored in vials at -20 °C between the conditioning experiments.
PER-conditioning Experiment
To find a suitable concentration of citral and linalool, a series of preliminary tests using differential conditioning were carried out. Dilutions of up to 1:1500 were tested. The preliminary tests were performed the same way as the main experiment, described in the remaining part of this subsection. The dilution of 1:1300 (equivalent to a concentration of 0.69 µg/µL for citral and 0.66 µg/µL for linalool) was chosen as only one third of the workers exhibited a behavioral response when presented with the diluted extract. Higher dilutions were deemed unsuitable for the experiment as they would provide insufficient data for the analysis.
For the differential conditioning (referred to as main experiment from now on), two stimulus combinations were used:
-
citral (dilution 1:1300) 5 µl as Cs + and linalool (dilution 1:1300) 5 µl as Cs-
-
Extract from Varroa-parasitized brood 5 µl as Cs + and the solvent isopropanol 5 µl as Cs-
As all the extracts contained isopropanol as a solvent, they were left to dry after being applied on the filter paper (including isopropanol as Cs-). This was done to ensure that the stimuli would not be overlayed by the smell of the solvent. A total of 15 bees from each colony were conditioned per stimulus combination for a total of 240 worker bees tested from each origin (VSH-selected and non-selected line). Each bee was conditioned using only one of the two stimulus combinations.
Parallel to the main conditioning experiment, a reversed differential conditioning was performed to assess potential differences in the salience of the stimuli used throughout the experiment. The stimulus combinations used in the main experiment were swapped:
-
Linalool (dilution 1:1300) 5 µl as Cs + and citral (dilution 1:1300) 5 µl as Cs-
-
Isopropanol 5 µl as Cs + and the extract from Varroa-parasitized brood 5 µl as Cs-.
The reversed conditioning was performed as described for the main experiment. Twenty workers were tested per stimulus combination and subsequently compared to the same number of workers conditioned with citral and Varroa-parasitized brood extract as Cs + .
During the main experiment, a total of 120 workers were conditioned per stimulus combination (citral as Cs + , Varroa-parasitized brood extract as Cs +) (Table 1). The bees were tested in groups of ten. Each group comprised individuals from different colonies. Before conditioning, worker bees were gathered from the brood frame in the test hive. The bees were shortly cooled down at -20 °C until they stopped moving. Subsequently, they were strapped in small metal tubes using paraffin tape so that the body was immobilized without the movement of the head and mouthparts being constricted. The worker bees were placed in an incubator (34 °C) to regain their physiological temperature after the cooling. The willingness of the worker bees to stretch their proboscis was examined by presenting them with a 50% sugar solution on a toothpick. One of the worker’s antennae was touched with a drop of the sugar solution which resulted in extension of the proboscis. Those workers that did not respond were not included in the conditioning.
As the solvent isopropanol was present in both Cs + and Cs-, only workers who were able to perceive the brood components, would sense the difference between both stimuli (Ivanova and Bienefeld 2021). The presentation of stimuli was conducted using pieces of filter paper and tweezers. The bees’ antennae were touched three times with a piece of untreated filter paper before the start of the experiment. This was done to avoid the extension of the proboscis due to a mechanical irritation rather than a response to the presented stimulus. During the stimuli presentation, both antennae were touched with the filter paper. The direct contact ensured the perception of both volatile and the non-volatile compounds, which are usually emitted by the distressed parasitized brood (Mondet et al. 2016; McAfee et al. 2018; Wagoner et al. 2020). A conditioned stimulus Cs + , the extract of Varroa-parasitized brood or citral, was paired with a 50% sugar solution (unconditioned stimulus Us). Additionally, an unreinforced stimulus, isopropanol or linalool, was presented without a reward (Cs-). The conditioning consisted of six trials in the following order: Cs + , Cs-, Cs-, Cs + , Cs + , Cs-. The intertrial-interval was between 4–5 min. Each worker bee was given 20 s to acclimate to the experimental surroundings, before a six second presentation of the stimulus. The presentation of the reward overlapped the last three seconds of the Cs + . The Us was presented by touching a drop of sucrose to the antennae without contaminating the filter paper carrying the Cs + . After completing the six trials, each worker bee was tested for its conditioning outcome through the presentation of the two stimuli (Cs + und Cs-) without the reward (unrewarded tests). If the conditioning was successful, the workers stretched their mouthparts to the presentation of the Cs + but not to the Cs-.
The experiments with the two odor combinations were swapped each day in order to exclude daytime biases. An exhaust system was used to remove any residual odors during the experiment. The toothpicks used for the presentation of the sugar reward were replaced before the beginning of each trial to avoid the accumulation of sugar. While wooden toothpicks give off a wooden odor, we assumed that the interference with the conditioning performance would be minimal as they were only used for the presentation of the reward. If workers were to form an association between the wooden odors and the sugar solution, they would not extend their proboscis when presented only with the Cs + during the unrewarded tests.
Worker bees which extended their proboscis during the first conditioning trial were excluded from the experiment as they were considered “spontaneous responders” which might have had prior contact with the stimuli used during the experiment or exhibit a heightened appetitive motivation that triggered PER to neutral stimuli (Matsumoto et al. 2012). Worker bees that stopped responding to the Us during the course of the conditioning were also excluded as they would also not shown any response during the unrewarded trials.
Statistical Analysis
Reversed Differential Conditioning
The acquisition during the reversed differential conditioning experiment was analyzed and compared to the stimuli as presented in the main experiment using a Chi-Square test (two-sided) with an alpha level of 0.05. The exact significance was reported.
Perception Speed and Conditioning Outcome
Using the data from the main experiment’s conditioning trials, the worker bees’ perception speed was analyzed with the help of a generalized linear mixed model with a logit function (GLMM) in SPSS V.25. The alpha level was set at 0.05. All reported p-values were two-sided. The parameters “VSH-selected line/non-selected line” and age during the experiment (< 6 days, 6–7 days, > 7 days) were set as fixed factors in the GLMM (see suppl. Tab. S1). The age groups were chosen in such a matter, so that the number of workers in each age group was similar. The colony effect was set as a random factor. In order to display the differences in the worker bees’ perception speed to the two highly diluted stimuli, three time points were defined. The extension of the proboscis to the Cs + before the presentation of the reward was considered a correct answer. For the Cs- a correct answer was defined as “no proboscis extension”.
-
Time point 1: Worker bees gave correct answers starting from trial No. 4 (Cs + , Cs-, Cs-, Cs + , Cs + , Cs-) and during the unrewarded trials (Cs + , Cs-)
-
Time point 2: Worker bees gave correct answers starting from trial No. 5 (Cs + , Cs-, Cs-, Cs + , Cs + , Cs-) and during the unrewarded trials (Cs + , Cs-)
-
Time point 3: Worker bees gave non-consecutive correct answers during the trials. The unrewarded tests were also correctly answered. (Cs + , Cs-, Cs-, Cs + , Cs + , Cs-) (Cs + , Cs-)
It was assumed that workers who made mistakes during the last three trials of the conditioning possessed an inferior discrimination ability than individuals that perceived the conditioning stimulus at Time point 1. Furthermore, to estimate the conditioning success of the two lines (VSH-selected/non-selected line) while taking the perception speed into account, a Kaplan–Meier estimator with a Log Rank function was performed. The significance level was set at 0.05 (two-sided).
Worker bees which showed no reaction during the conditioning and gave a positive answer only during the unrewarded tests, were not considered successful, as it was unsure whether the response occurred coincidentally. A proboscis extension was recorded as “1”, no behavioral response was documented as “0”.
Results
Floral Stimuli
Reversed Differential Conditioning
Compared to linalool as Cs + , workers tested with citral as Cs + exhibited a significantly higher proboscis extension frequency during the conditioning trials. Workers tended to generalize more at trials 2–3 when citral was used as Cs + and exhibited significantly higher number of proboscis extensions during trials three to six (Fig. 1 and suppl. Tab. S2). The generalization was stronger at the beginning of the conditioning and decreased with each trial. At trial six a slight decrease (40%) in proboscis extension frequency was observed when workers were presented with the Cs- (linalool) than at trial three (45%) (Fig. 1).
Acquisition curves and unrewarded tests for conditioning with citral as Cs + (black line) and linalool as Cs + (grey line). (a) Proboscis extension frequencies for citral as Cs + / linalool as Cs- and linalool as Cs + / citral as Cs- are shown in percent for each trial. Significant differences are marked with an asterisk. The alpha-level is set at 0.05. Per conditioning experiment (citral as Cs + / linalool as Cs- and linalool as Cs + / citral as Cs-) the same number of worker bees were used (N = 20). (b) Proboscis extension frequencies during the unrewarded tests are shown in percent. No significant differences were observed
During the unrewarded tests no significant difference in the proboscis extension frequency for citral and linalool was observed (unrewarded Cs + : χ2(1; N = 40) = 0.91; p = 0.53; unrewarded Cs-: χ2(1; N = 40) = 2.9; p = 0.49). Workers tested with citral exhibited higher numbers of proboscis extensions during the unrewarded tests although the difference was not significant. With regard to the results, citral and linalool were considered perceptually similar with citral posing a more potent stimulus at the chosen dilution.
Perception Speed and Conditioning Outcome for Both Origins (Citral as Cs + , Linalool as Cs-)
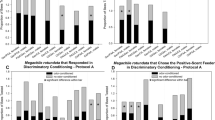
During the main conditioning experiment with the flower substances, no significant differences in stimulus perception between the two lines were observed. The VSH-selected line exhibited a slightly higher percentage of worker bees that perceived the difference between the two stimuli (citral as Cs + and linalool as Cs-) at the earliest time point (Time point 1) than the non-selected line (VSH-selected line: 15%; non-selected line: 10.8%) (see Fig. 2, suppl. Tab. S3). The relative increase of stimulus perception by the VSH-selected line was 39% (equivalent to 5 worker bees more than the non-selected line). Worker bees that perceived citral at one of the later conditioning time points, also showed no origin-related differences in perception (VSH-selected line/non-selected line). The perception ability of the tested workers did not differ with age (see suppl. Tabs. S4 and S5). The colony effect also had no significant influence on stimulus detection (see suppl. Fig. S F1).
Number of workers, that are able to perceive the Cs + (citral, dilution 1:1300), in their corresponding speed group. Displayed are the two lines – VSH-selected line (white) and the non-selected line (black). The columns show the number of workers which successfully perceived the Cs + at one of the three time points. Each worker is listed in only one group. Standard error is displayed for each time point and group
A Kaplan–Meier curve was created to display the perception ability of the participating worker bees (see Fig. 3). The overall conditioning outcome of the two groups (VSH-selected line and non-selected line) shown during the unrewarded tests with citral as Cs + and linalool as Cs- exhibited no statistically significant difference (Kaplan–Meier estimator, Long rank test, χ2(1; N = 240) = 0.60, p = 0.438).
Kaplan–Meier perception curve for the flower odors. Citral (dilution 1:1300) was used as Cs + and linalool (dilution 1:1300) as Cs-. The cross at the end of each line represents the end of the conditioning for all subjects of the corresponding group. The three vertical lines represent Time points 1, 2 and 3. The collective perception of the bees in each group is displayed on the y-axis in percent. The two groups are presented separately: VSH-selected line (N = 120; red color), non-selected line (N = 120; blue color)
Varroa-parasitized Brood Extract
Reversed Differential Conditioning
Worker bees in the reversed differential conditioning experiment with isopropanol as Cs + discriminated well between the solvent isopropanol and the extract of Varroa-parasitized brood. No differences were found in the ability to discriminate between substances, regardless of which substance (brood extract or solvent) was chosen as the conditioning stimulus Cs + (Fig. 4 and suppl. Table S7). The unrewarded tests also did not provide significant differences in proboscis extension responses (unrewarded Cs + : χ2(1; N = 40) = 0.10; p = 1.0; unrewarded Cs-: χ2(1; N = 40) = 0.37; p = 1.0) between the two substances. These results led us to believe that none of the two substances posed as a stronger conditioning stimulus for workers during the experiment.
Acquisition curves and unrewarded tests for conditioning with isopropanol as Cs + (black line) and Varroa-parasitized brood extract as Cs + (grey line). (a) Proboscis extension frequencies for isopropanol as Cs + / Varroa-parasitized brood extract as Cs- and Varroa-parasitized brood extract as Cs + / isopropanol as Cs- are shown in percent for each trial. The alpha-level is set at 0.05. Per conditioning experiment (isopropanol as Cs + / Varroa-parasitized brood extract as Cs- and Varroa-parasitized brood extract as Cs + / isopropanol as Cs-) the same number of worker bees were used (N = 20). No significant differences were observed. (b) Proboscis extension frequencies during the unrewarded tests are shown in percent. The alpha-level is set at 0.05. No significant differences were observed
Perception Speed and Conditioning Outcome of Both Origins (Varroa-Parasitized Brood Extract as Cs + , Isopropanol as Cs-)
At the earliest possible time point, Time point 1, 10% more VSH-selected line bees (12 workers) perceived the Varroa-parasitized-brood extract than the non-selected line (see Fig. 5, suppl. Tab. S8). This percentage difference corresponds to 133% relative increase of the VSH-selected line’s response rate. The differences were statistically significant (GLMM, p = 0.027; CI: 0.11; 1.77). Moreover, the VSH-selected line had 2.6 times higher odds of perceiving the Cs + at Time point 1 than the non-selected line (OR = 2.6; CI: 1.12; 5.89) (see suppl. Tab. S9).
Number of worker bees, that are able to perceive the Cs + (Varroa-parasitized-brood extract), shown in their corresponding speed group. Displayed are the two lines – VSH-selected line (white) and the non-selected line (black). The columns show the number of workers which successfully perceived the Cs + at one of the three time points. Each worker is listed in only one group. Standard error is displayed for each time point and group
Worker bees from both the VSH-selected and non-selected lines that perceived the Varroa-parasitized brood extract at Time points 2 and 3 performed similarly (see suppl. Tabs. S10 and S11). Again, there was no difference between the three age groups in terms of the ability of the worker bees’ perception of the extract. Similar to the conditioning with citral and linalool, the colony effect also had no significant influence on stimulus detection (see suppl. Fig. S F2).
The VSH-selected and non-selected lines exhibited a difference in their overall conditioning outcome during the unrewarded tests. The VSH-selected line displayed a higher percentage of worker bees (34%) which were able to perceive the extract of Varroa-parasitized brood (see Fig. 6) than the non-selected line (23%). The difference was significant (Kaplan–Meier estimator, Long rank test, χ2(1; N = 240) = 3.97, p = 0.046).
Kaplan–Meier perception curve for the Varroa-parasitized-brood extract. The Varroa-parasitized-brood extract was used as Cs + , the solvent isopropanol as Cs-. The cross at the end of each line represents the end of the conditioning for all subjects of the corresponding group. The three vertical lines represent Time points 1, 2 and 3. The cumulative perception of each line is displayed on the y-axis in percent. The two groups are displayed separately: VSH-selected line (N = 120; red color), non-selected line (N = 120; blue color)
Discussion
In the course of this work two sets of experiments were carried out. The salience of the substances used throughout the experiments was assessed using a reversed differential conditioning with groups of 20 workers per stimulus combination. Furthermore, during the main experiment, a total of 240 workers – 120 from the VSH-selected line and 120 from the non-selected line – were conditioned per stimulus combination.
Because the PER response does not measure the sensitivity of an individual bee per se, but the learning behavior to a stimulus, we hypothesized that there would be no learning success even with large differences in learning ability, if the stimulus is not recognized. As sensitivity towards different chemical cues plays a central role in hygienic behavior (Schöning et al. 2012; Mondet et al. 2015, 2021; Wagoner et al. 2021), the learning behavior of the worker bees was used to determine whether low concentrations of the stimuli are perceived at all and thus could be regarded as marker for perception ability and olfactory sensitivity.
All three research questions could be answered. Although the chosen experimental setup did not examine the ability of workers to differentiate between healthy and parasitized brood, it displayed workers’ ability to perceive the complex chemical bouquet of Varroa-parasitized brood cues at a very low concentration. No difference in salience was observed between the extract of Varroa-parasitized brood and the solvent isopropanol during the reversed differential conditioning, further strengthening this observation.
The results of the main experiment additionally indicated an enhanced specific sensitivity in the VSH-selected line towards chemical cues emitted by the brood, which can play a role in detection of Varroa-parasitization. When tested with a low concentration of the Varroa-parasitized brood extract, workers selected for VSH exhibited a significantly higher perception ability and a higher percentage of stimulus recognition (Cs +) at the earliest possible time point compared to the non-selected line.
Unlike the observations from the conditioning with the Varroa-parasitized brood extract and isopropanol, citral and linalool exhibited significant differences in the proboscis extension frequencies during most trials of the reversed conditioning. Citral posed as more salient compared to linalool at the dilution (1:1300) used in the course of this work. During the main experiment, the highly diluted floral extract citral was perceived equally well by both lines. The speed of perception for citral was also comparable for the two lines.
Perception Ability of Worker Bees Towards Flower Substances
Odor detection is an important part of food and host selection in invertebrates and mammals (Visser 1986; Masson and Mustaparta 1990; Firestein 2001). The ability to form an association and gather experience from previous foraging decisions is a result of long-lasting natural selection in the honey bee, as foraging behavior acts as a major determinant for the survival of both the individual and the colony (Kramer 2001; Page et al. 2006). Olfactory generalization is considered crucial for foragers’ ability to find suitable food sources with varying volatile release (Sandoz et al. 2001). This ability allows animals to extend a behavior from a particular stimulus to another, novel stimulus, which is perceived similarly enough (Shepard 1987). Especially molecules with a similar carbon length and chemical group are subjected to high generalization (Sandoz 2011).
The ability to distinguish between different odorants is also dependent on stimulus concentration (Getz and Smith 1991; Wright 2004). During our preliminary tests, we observed a great decrease in the behavioral responses to both substances (citral and linalool) when using a dilution of more than 1:1300 (equivalent to a concentration of 0.69 µg/µL solution) for the differential conditioning. Only a third of the worker bees used in the conditioning discriminated between stimuli of this particular dilution by touching the filter paper with the antennae or sensing the emitted odor via molecules in the air, therefore we chose not to dilute our probe any further in order to gather sufficient data on the differences in discrimination ability between the two lines. Nevertheless, from the data gathered during the preliminary tests, we suspected that the conditioning threshold for citral using a tactile presentation lies in the range of 1:1500 (~ 0.6 µg/µL).
In the course of the reversed differential conditioning, we observed a generalization between citral and linalool when citral was rewarded. This was not the case when linalool was used as Cs + . One possible reason for the generalization could be the similar carbon chain length of their molecular structures – C10H16O (citral) and C10H18O (linalool). Another reason could be the fact that citral not only plays a role as a flower odor but is also a compound found in secretions of the Nasonov gland (Butler and Calam 1969; Getz and Smith 1991). Social pheromones are described as producing higher generalization as general odors which would suggest that biological value influences generalization (Sandoz et al. 2001). This could explain the overall higher proboscis extension response frequency when citral was used as Cs + . Shearer and Boch (1966) described citral as a minor compound of the Nasonov pheromone that increases the attractiveness of geraniol – one of the major components – when both are presented together. On its own, citral was far less attractive than geraniol or the Nasonov pheromone itself (Shearer and Boch 1966; Williams et al. 1981). It could therefore be argued that citral’s biological value is given only as part of the mixture and the experimental results display merely a difference in attractiveness between two floral substances.
When both VSH-selected line worker bees and non-selected line worker bees were trained with the highly diluted floral compounds during the main experiment, they exhibited a similar olfactory sensitivity and discrimination ability. The VSH-selected line showed a 14% relative improvement of perception. Nevertheless, this difference was not significant. Unlike previous studies like those by Masterman et al. (2000) and Chakroborty et al. (2015), where undiluted odors were used to observe differences in perception, we hypothesized that high concentrations pose an easy task for the test subjects and provide information on overall perception ability but give no feedback on olfactory sensitivity. Such information can only be displayed by using concentrations near the threshold limit for eliciting a behavioral response (Laska 2000). We can now add to the previous studies’ conclusions and confirm that the olfactory sensitivity and discrimination ability of hygienic and non-hygienic lines does not significantly differ when low concentrations near the perception threshold of citral and linalool are used.
PerceptionAbility of Worker Bees Towards the Varroa-parasitized Brood Extract
During the conditioning with a low concentration of the Varroa-parasitized-brood extract, a different picture than with the flower extracts was observed. The VSH-selected line exhibited a significantly stronger tendency of perceiving the complex bouquet of Varroa-parasitized brood than the non-selected line, with a relative improvement of 133% in perception.
Previous studies proved an important step in describing the improvements in hygienic behavior caused by breeding efforts (Ivanova and Bienefeld 2021). Masterman et al. (2001, 2000) described a better performance of hygienic lines when conditioned to the strong stimulus of chalkbrood diseased brood. Chakroborty et al. (2015) used live pupae parasitized by V. destructor to assess differences in the perception ability of worker bees from VSH-selected and non-selected lines and described a “slightly better performance” of the VSH bees as well. This tendency of VSH workers to better perceive the cues connected to a V. destructor parasitization are likely a result of proteome differences in the central nervous system and the antennae of worker bees (Mondet et al. 2015; Hu et al. 2016). Mondet et al. (2015) conducted a differential gene expression on the antennae of bees selected for VSH and non-VSH. Genes connected to defense responses were over-expressed in VSH-bees’ antennae (Mondet et al. 2015). In the mushroom bodies, proteins connected to neuronal sensitivity by activation of synaptic vesicles and calcium channels were upregulated in VSH workers (Hu et al. 2016). Moreover, hygienic bees were shown to have lower stimulus thresholds for olfactory and behavioral responses than non-hygienic bees (Masterman et al. 2001). Boutin et al. (2015) suspected that non-hygienic bees have an over-expression of cytochrome P450, an enzyme that participates in the degradation of odorant pheromones. An over-expression could lead to the removal of stimuli before the hygienic behavior can be initiated and thus influence the worker bee’s olfactory capability.
Compared to Masterman et al. (2001) and Chakroborty et al. (2015), the brood extract during our conditioning experiments contained only a fraction of the stimulus intensity (for comparison: 0.02 brood equivalents versus a whole parasitized pupa) so as to approach the perception threshold of worker bees as much as possible while still eliciting a behavioral response. Furthermore, we used a tactile presentation which is closer to the natural perception of Varroa-parasitized brood in the colony, compared to the air stream presentation used by Chakroborty et al. (2015). While it is possible that tested workers might have been exposed to higher concentrations of the extract through the direct contact with the filter paper compared to the amounts of the extract delivered only via an air stream, we hypothesized that the very low concentration of the extract would nevertheless provide a more difficult task for the workers than previously done by Chakroborty et al. (2015). Our aim was to mimic reality as closely as possible, considering that worker bees in the colony must recognize subtle brood distress signals, superimposed by the odors of neighboring cells, through the closed cell caps. While discrimination between the extract of Varroa-parasitized brood and the cuticular profile of healthy brood was not tested in the course of this work, the results from the main conditioning experiment (Varroa-parasitized brood extract as Cs +) nevertheless showed that workers can perceive the low concentration of Varroa-parasitized brood extract and clearly distinguish it from the solvent. This observation was strengthened by the fact, that no difference in salience was present between extract and the solvent isopropanol during the reversed conditioning. The differences in discriminatory ability between the two lines made during the main experiment are therefore not due to contrasts in stimulus intensity but a result of selection breeding.
Speed of Perception
Both lines (VSH-selected and non-selected line) showed similar numbers of worker bees with a positive conditioning outcome for the highly diluted citral. Although the VSH-selected line exhibited a 39% relative improvement of perception at Time point 1 compared to the non-selected line, the difference was non-significant.
During the experiment with the Varroa-parasitized brood extract, both lines displayed worker bees which are capable of early perception. While most of the worker bees from both lines exhibited similar discriminatory ability and perceived the extract at Time point 3, only a third of all workers displayed superior olfactory sensitivity. At the earliest possible time point (Time point 1), VSH-selected line showed 2.6 times higher odds of perceiving the Varroa-parasitized brood extract than the non-selected line, complementing a relative improvement of perception of 133%. A possible explanation for the different numbers of worker bees exhibiting fast perception in both groups is the aforementioned difference in stimulus threshold. While both hygienic and non-hygienic hives exercise hygienic behavior, the latter remove diseased brood less efficiently (Arathi et al. 2000; Arathi and Spivak 2001). VSH-selected bees might be responding to stimuli faster thanks to a difference in the expression of genes compared to non-hygienic bees (Navajas et al. 2008; le Conte et al. 2011; Hu et al. 2016; Gempe et al. 2016).
It could be argued that the observed results are caused by a higher "sucrose responsiveness" of the VSH line and not by a superior olfactory sensitivity. However, in our experiment the workers from both lines (hygienic and non-hygienic) displayed similar reactions to sucrose during the conditioning, suggesting that the enhanced ability of hygienic bees to perceive diseased brood cues during conditioning experiments is independent from the bees’ sucrose responsiveness. Rather, it appears that the VSH breeding efforts produce higher olfactory sensitivity in worker bees, leading to the higher sensitivity to low concentrations of the Varroa-parasitized brood extract as observed. Learning performance and speed were no selection criteria for the creation of the VSH-selected line. The selection criterion used was the reaction to parasitized brood, whose chemical profile is known to deviate from that of healthy brood (Mondet et al. 2021). These observations are consistent with Goode et al. (2006), who suggested that the high sensitivity towards pathological cues does lead to a quicker and more efficient detection and removal of parasitized brood.
Lapidge et al. (2002) suggested that hygienic behavior is a quantitative trait whose differential expression leads to variations in each hive’s performance and even between bees in the same hive. Indeed, Gramacho and Spivak (2003) observed differences in the olfactory sensitivity in bees from the same hive and of the same age that were performing hygienic behavior. During their PER conditioning experiment, worker bees that initiated uncapping behavior exhibited greater olfactory sensitivity than bees which were engaged only in removing the brood. This variation is likely a consequence of the queen mating with several drones from different colonies. In contrast, in our experiments the VSH-selected line was created exclusively through artificial insemination with sperm from several drones coming from one colony, resulting in less gene dispersion within the colonies.
With the results of our experiments in mind, we anticipate that the worker bees with the highest perception speed towards the extract of Varroa-parasitized brood at Time point 1, could, in fact, be the most sensitive ones and most likely to elicit uncapping behavior. The difference in the number of workers with a higher olfactory sensitivity is most probably a result of the Varroa-resistance breeding efforts. More studies are needed to further strengthen this hypothesis. This could be done by testing worker bees with a high perception speed towards the extract of Varroa-parasitized brood for their uncapping activity on a brood frame, artificially infested with V. destructor. As workers with faster perception for the Varroa-parasitized brood extract were also present in the non-selected line but in smaller numbers, we expect to exhibit a difference in the uncapping activity between the two origins.
Enhanced Specific Olfactory Sensitivity
The results from our experiments suggest breeding efforts can enhance bees’ olfactory sensitivity and discrimination ability to chemical cues emitted from the brood in connection to a V. destructor parasitization. The VSH-selected line displayed specific higher sensitivity towards the extract of Varroa-parasitized brood compared to the non-selected line. This specific sensitivity was characterized by a faster (at the earliest time point) and generally higher perception of the complex chemical blend emitted by the brood, even in small quantities and low concentrations. The sensitivity to flower extracts was comparable to that of the non-selected line.
Specialization to ecologically relevant stimuli has been observed in countless species. It supplies the nervous system with valuable information, allowing animals to respond appropriately to a given situation (Hansson and Stensmyr 2011). Olfaction plays an important role in most insects (Dethier 1947), and changes in the olfactory system can enhance the fitness and breeding success (Hansson and Stensmyr 2011). In Drosophila sechellia, for instance, increased numbers of one type of olfactory sensillum allow the fly to specialize in one type of fruit that is toxic to other drosophilids (Hansson and Stensmyr 2011; Linz et al. 2013). Mosquitoes of the Culex taxa possess high selectivity and sensitivity towards nonanal—a semiochemical characteristic for birds and humans — allowing the insects to detect their hosts from a long range (Syed and Leal 2009). For honey bees, the ability to detect disease-specific cues is a vital part of hygienic behavior and social immunity. Workers exhibiting higher olfactory sensitivity to abnormal brood initiate its removal thus prolonging the survival of the colony (Gramacho and Spivak 2003).
While the development of resistant honey bee populations based on increased VSH can occur naturally (Panziera et al. 2017), breeding efforts have also been shown to successfully increase hygienic behavior (Pérez-Sato et al. 2009). Indeed, enhanced hygienic behavior is correlated with various changes to the proteome of the olfactory system (Parker et al. 2012), particularly the expression of different proteins such as Odorant binding protein, VAMP, Calcyclin Binding Protein, which are connected to signal transduction in the antennae (Guarna et al. 2015). As Gempe et al. (2016) describe, an over-represented signal transduction can be seen in the brain of highly hygienic bees. Furthermore, bees tolerant to V. destructor also display an up-regulation of genes connected to neuronal excitability (Navajas et al. 2008). These genes might participate in the increase of responsiveness to environmental stimuli and lead to engagement in hygienic behavior (Navajas et al. 2008). While the PER conditioning can be used to estimate for differences in discrimination ability and sensitivity, we suspect that the measured differences may not adequately reflect the complete potential of bees with respect to their hygienic behavior to Varroa-parasitized brood. One reason for this can be the stress caused by the conditioning itself. Furthermore, cues other than those emitted from the brood – like thermal cues – could play a supplementary role in the decision to uncap a brood cell (Bauer et al. 2018). In an extensive experiment conducted at the Institute for Bee Research Hohen Neuendorf, the worker bees of the VSH-selected line started uncapping 8-times more Varroa-parasitized cells than bees of the non-selected control line (Bienefeld, in preparation). Previous research further displayed strong maternal and additive genetic effects for the manifestation of VSH (Ivanova and Bienefeld 2021). The results shown by the worker bees in this experiment demonstrate enhancements of the VSH-selected line’s specific olfactory sensitivity towards cues emitted by the brood caused by resistance breeding.
Conclusion
Our findings further deepen the knowledge of VSH and provide valuable information on the effects of breeding for Varroa-resistance. The difference in perception speed shown by the VSH-selected line during the PER conditioning experiment is most likely based on a lower stimulus threshold for olfactory and behavioral responses. However, more research is needed to optimize the methodology of assessing sensitivity to the relevant stimuli and to determine whether other influencing variables are further drivers of hygienic behavior towards sick brood, beyond characteristics that control olfactory sensitivity.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arathi HS, Burns I, Spivak M (2000) Ethology of hygienic behavior in the honey bee Apis mellifera L. (Hymenoptera: apidae): behavioural repertoire of hygienic bees. Ethology 106:365–379. https://doi.org/10.1046/j.1439-0310.2000.00556.x
Arathi HS, Spivak M (2001) Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee, Apis mellifera L. Anim Behav 62:57–66. https://doi.org/10.1006/anbe.2000.1731
Bauer D, Wegener J, Bienefeld K (2018) Recognition of mite-infested brood by honeybee (Apis mellifera) workers may involve thermal sensing. J Therm Biol 74:311–316. https://doi.org/10.1016/j.jtherbio.2018.04.012
Bienefeld K, Reinsch N, Thakur RK (2001) Selection for uncapping of Varroa infested brood cells in the honeybee (Apis mellifera). In: Proc. 37th Int. Apic. Congr. Apimondia Publishing House, Durban, South Africa
Bienefeld K, Zautke F, Gupta P (2015) A novel method for undisturbed long-term observation of honey bee (Apis mellifera) behavior – illustrated by hygienic behavior towards Varroa infestation. J Apic Res 54:541–547. https://doi.org/10.1080/00218839.2016.1174465
Bitterman ME, Menzel R, Fietz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119. https://doi.org/10.1037/0735-7036.97.2.107
Boutin S, Alburaki M, Mercier P-L et al (2015) Differential gene expression between hygienic and non-hygienic honeybee (Apis mellifera L.) hives. BMC Genom 16:500. https://doi.org/10.1186/s12864-015-1714-y
Butler CG, Calam DH (1969) Pheromones of the honey bee—The secretion of the Nassanoff gland of the worker. J Insect Physiol 15:237–244. https://doi.org/10.1016/0022-1910(69)90271-6
Chakroborty NK, Bienefeld K, Menzel R (2015) Odor learning and odor discrimination of bees selected for enhanced hygienic behavior. Apidologie 46:499–514. https://doi.org/10.1007/s13592-014-0342-x
Dethier VG (1947) Chemical insect attractants and repellents. The Blakiston Co., Philadelphia and Toronto, pp 289
Dietemann V, Pflugfelder J, Anderson D et al (2012) Varroa destructor: research avenues towards sustainable control. J Apic Res 51:125–132. https://doi.org/10.3896/IBRA.1.51.1.15
Firestein S (2001) How the olfactory system makes sense of scents. Nature 413:211–218. https://doi.org/10.1038/35093026
Gempe T, Stach S, Bienefeld K et al (2016) Behavioral and molecular studies of quantitative differences in hygienic behavior in honeybees. BMC Res Notes 9:474. https://doi.org/10.1186/s13104-016-2269-y
Genersch E, von der Ohe W, Kaatz H et al (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41:332–352. https://doi.org/10.1051/apido/2010014
Getz WM, Smith KB (1991) Olfactory perception in honeybees: concatenated and mixed odorant stimuli, concentration, and exposure effects. J Comp Physiol A 169:215–230. https://doi.org/10.1007/BF00215869
Giurfa M (2008) Behavioral and neural analysis of associate learning in the honeybee. In: Byrne JH (ed) Learning and memory: a comprehensive reference. Elsevier, Oxford, pp 561–585
Giurfa M, Malun D (2004) Associative mechanosensory conditioning of the proboscis extension reflex in honeybees. Learn Mem 11:294–302. https://doi.org/10.1101/lm.63604
Goode K, Huber Z, Mesce KA, Spivak M (2006) Hygienic behavior of the honey bee (Apis mellifera) is independent of sucrose responsiveness and foraging ontogeny. Horm Behav 49:391–397. https://doi.org/10.1016/j.yhbeh.2005.08.007
Gramacho KP, Spivak M (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav Ecol Sociobiol 54:472–479. https://doi.org/10.1007/s00265-003-0643-y
Guarna MM, Melathopoulos AP, Huxter E et al (2015) A search for protein biomarkers links olfactory signal transduction to social immunity. BMC Genom 16:63. https://doi.org/10.1186/s12864-014-1193-6
Hansson BS, Stensmyr MC (2011) Evolution of insect olfaction. Neuron 72:698–711. https://doi.org/10.1016/j.neuron.2011.11.003
Harbo JR, Harris JW (2005) Suppressed mite reproduction explained by the behaviour of adult bees. J Apic Res 44:21–23. https://doi.org/10.1080/00218839.2005.11101141
Hu H, Bienefeld K, Wegener J et al (2016) Proteome analysis of the hemolymph, mushroom body, and antenna provides novel insight into honeybee resistance against Varroa infestation. J Proteome Res 15:2841–2854. https://doi.org/10.1021/acs.jproteome.6b00423
Ivanova I, Bienefeld K (2021) Suitability of drone olfactory sensitivity as a selection trait for Varroa-resistance in honeybees. Sci Rep 11:17703. https://doi.org/10.1038/s41598-021-97191-w
Jacques A, Laurent M, Ribière-Chabert M et al (2017) A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PloS One 12:e0172591. https://doi.org/10.1371/journal.pone.0172591
Kim SH, Mondet F, Hervé M, Mercer A (2018) Honey bees performing Varroa sensitive hygiene remove the most mite-compromised bees from highly infested patches of brood. Apidologie 49:335–345. https://doi.org/10.1007/s13592-017-0559-6
Kramer DL (2001) Foraging Behavior. In: Fox CW, Roff DA (eds) Evolutionary ecology: concepts and case studies. Oxford University Press, pp 232–246
Lapidge KL, Oldroyd BP, Spivak M (2002) Seven suggestive quantitative trait loci influence hygienic behavior of honey bees. Sci Nat 89:565–568. https://doi.org/10.1007/s00114-002-0371-6
Laska M (2000) “Microsmatic” primates revisited: olfactory sensitivity in the squirrel monkey. Chem Senses 25:47–53. https://doi.org/10.1093/chemse/25.1.47
le Conte Y, Alaux C, Martin JF et al (2011) Social immunity in honeybees (Apis mellifera): transcriptome analysis of Varroa-hygienic behavior. Insect Mol Biol 20:399–408. https://doi.org/10.1111/j.1365-2583.2011.01074.x
Linz J, Baschwitz A, Strutz A et al (2013) Host plant-driven sensory specialization in Drosophila erecta. Proc Royal Soc B 280:20130626. https://doi.org/10.1098/rspb.2013.0626
Locke B (2016) Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47:467–482. https://doi.org/10.1007/s13592-015-0412-8
Martin C, Provost E, Bagnères AG et al (2002) Potential mechanism for detection by Apis mellifera of the parasitic mite Varroa destructor inside sealed brood cells. Physiol Entomol 27:175–188. https://doi.org/10.1046/j.1365-3032.2002.00284.x
Martin SJ, Highfield AC, Brettell L et al (2012) Global honey bee viral landscape altered by a parasitic mite. Science (1979) 336:1304–1306. https://doi.org/10.5061/dryad.d54cc
Masson C, Mustaparta H (1990) Chemical information processing in the olfactory system of insects. Physiol Rev 70:199–245. https://doi.org/10.1152/physrev.1990.70.1.199
Masterman R, Ross R, Mesce M, Spivak M (2001) Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J Comp Physiol A 187:441–452. https://doi.org/10.1007/s003590100216
Masterman R, Smith BH, Spivak M (2000) Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J Insect Behav 13:87–101. https://doi.org/10.1023/A:1007767626594
Matsumoto Y, Menzel R, Sandoz JC, Giurfa M (2012) Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods 211:159–167. https://doi.org/10.1016/j.jneumeth.2012.08.018
McAfee A, Chapman A, Iovinella I et al (2018) A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.). Sci Rep 8:5719. https://doi.org/10.1038/s41598-018-24054-2
Mondet F, Alaux C, Severac D et al (2015) Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci Rep 5:10454. https://doi.org/10.1038/srep10454
Mondet F, Beaurepaire A, McAfee A et al (2020) Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Int J Parasitol 50:433–447. https://doi.org/10.1016/j.ijpara.2020.03.005
Mondet F, Blanchard S, Barthes N et al (2021) Chemical detection triggers honey bee defense against a destructive parasitic threat. Nat Chem Biol 17:524–530. https://doi.org/10.1038/s41589-020-00720-3
Mondet F, Kim SH, de Miranda JR et al (2016) Specific cues associated with honey bee social defense against Varroa destructor infested brood. Sci Rep 6:25444. https://doi.org/10.1038/srep25444
Nagaraja N, Bruckner D (2013) Olfactory learning and memory recall in drones of hive honeybee species. J Entomol Res 37:29–32
Navajas M, Migeon A, Alaux C et al (2008) Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom 9:1–11. https://doi.org/10.1186/1471-2164-9-301
Nazzi F, della Vedova G, D’-Agaro M (2004) A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35:65–70. https://doi.org/10.1051/apido:2003065
Oddie M, Büchler R, Dahle B et al (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8:7704. https://doi.org/10.1038/s41598-018-26001-7
Oddie M, Dahle B, Neumann P (2017) Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ 5:e3956. https://doi.org/10.7717/peerj.3956
Page RE, Peng C (2001) Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol 36:695–711. https://doi.org/10.1016/S0531-5565(00)00236-9
Page RE, Scheiner R, Erber J, Amdam Gv (2006) The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr Top Dev Biol 74:253–286. https://doi.org/10.1016/S0070-2153(06)74008-X
Panziera D, van Langevelde F, Blacquière T (2017) Varroa sensitive hygiene contributes to naturally selected Varroa resistance in honey bees. J Apic Res 56:635–642. https://doi.org/10.1080/00218839.2017.1351860
Parker R, Guarna MM, Melathopoulos AP et al (2012) Correlation of proteome-wide changes with social immunity behaviors provides insight into resistance to the parasitic mite, Varroa destructor, in the honey bee (Apis mellifera). Genome Biol 13:R81. https://doi.org/10.1186/gb-2012-13-9-r81
Pérez-Sato JA, Chline N, Martin SJ et al (2009) Multi-level selection for hygienic behavior in honeybees. Heredity (Edinb) 102:609–615. https://doi.org/10.1038/hdy.2009.20
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:96–119. https://doi.org/10.1016/j.jip.2009.07.016
Sandoz JC (2011) Behavioral and neurophysiological study of olfactory perception and learning in honeybees. Front Syst Neurosci 5:1–20. https://doi.org/10.3389/fnsys.2011.00098
Sandoz JC, Pham-Delègue MH, Renou M, Wadhams LJ (2001) Asymmetrical generalisation between pheromonal and floral odours in appetitive olfactory conditioning of the honey bee (Apis mellifera L). J Comp Physiol A 187:559–568. https://doi.org/10.1007/s003590100228
Schöning C, Gisder S, Geiselhardt S et al (2012) Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J Exp Biol 215:264–271. https://doi.org/10.1242/jeb.062562
Shearer DA, Boch R (1966) Citral in the Nassanoff pheromone of the honey bee. J Insect Physiol 12:1513–1521. https://doi.org/10.1016/0022-1910(66)90041-2
Shepard RN (1987) Toward a universal law of generalization for psychological science. Science (1979) 237:1317–1323
Smith BH, Burden CM (2014) A proboscis extension response protocol for investigating behavioral plasticity in insects: application to basic, biomedical, and agricultural research. J Vis Exp: e51057. https://doi.org/10.3791/51057
Spötter A, Gupta P, Mayer M et al (2016) Genome-wide association study of a Varroa-specific defense behavior in honeybees (Apis mellifera). J Hered 107:220–227. https://doi.org/10.1093/jhered/esw005
Spötter A, Gupta P, Nürnberg G et al (2012) Development of a 44K SNP assay focussing on the analysis of a Varroa-specific defence behaviour in honey bees (Apis mellifera carnica). Mol Ecol Resour 12:323–332. https://doi.org/10.1111/j.1755-0998.2011.03106.x
Swanson JAI, Torto B, Kells SA et al (2009) Odorants that induce hygienic behavior in honeybees: identification of volatile compounds in chalkbrood-infected honeybee larvae. J Chem Ecol 35:1108–1116. https://doi.org/10.1007/s10886-009-9683-8
Syed Z, Leal WS (2009) Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc Natl Acad Sci U S A 106:18803–18808. https://doi.org/10.1073/pnas.0906932106
Takeda K (1961) Classical conditioned response in the honey bee. J Insect Physiol 6:168–179. https://doi.org/10.1016/0022-1910(61)90060-9
Vareschi E (1971) Duftunterscheidung bei der Honigbiene – und Verhaltensreaktionen. Z Vgl Physiol 75:143–173. https://doi.org/10.1007/BF00335260
Vidal-Naquet N (2015) Honeybee veterinary medicine: Apis Mellifera L. 5M Publishing
Visser JH (1986) Host odor perception in phytophagous insects. Annu Rev Entomol 31:121–144. https://doi.org/10.1146/annurev.en.31.010186.001005
Wagoner K, Millar JG, Keller J et al (2021) Hygiene-eliciting brood semiochemicals as a tool for assaying honey bee (Hymenoptera: Apidae) colony resistance to Varroa (Mesostigmata: Varroidae). J Insect Sci 21. https://doi.org/10.1093/jisesa/ieab064
Wagoner K, Spivak M, Hefetz A et al (2019) Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Sci Rep 9:8753. https://doi.org/10.1038/s41598-019-45008-2
Wagoner KM, Millar JG, Schal C, Rueppell O (2020) Cuticular pheromones stimulate hygienic behavior in the honey bee (Apis mellifera). Sci Rep 10:7132. https://doi.org/10.1038/s41598-020-64144-8
Wagoner KM, Spivak M, Rueppell O (2018) Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae). J Econ Entomol 111:2520–2530. https://doi.org/10.1093/jee/toy266
Williams IH, Pickett JA, Martin AP (1981) The Nasonov pheromone of the honeybee Apis mellifera L. (Hymenoptera, Apidae). Part II. Bioassay of the components using foragers. J Chem Ecol 7:225–237. https://doi.org/10.1007/BF00995745
Wright GA (2004) Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera). Chem Senses 29:127–135. https://doi.org/10.1093/chemse/bjh016
Acknowledgements
We would like to thank Fred Zautke and Ivonne Kretschmann for providing the necessary bees and technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. We would also like to thank the German Federal Environmental Foundation (AZ 20019/596) for the funding.
Author information
Authors and Affiliations
Contributions
K.B. and I.I. designed the study. I.I. conducted the experiments, analyzed the data and wrote the paper. KB supervised the study and assisted with the interpretation of results and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no conflict of interests.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ivanova, I., Bienefeld, K. Apis mellifera Worker Bees Selected for Varroa-sensitive Hygiene Show Higher Specific Sensitivity and Perception Speed Towards Low Concentrations of Chemical Cues Emitted by the Brood. J Insect Behav 36, 96–112 (2023). https://doi.org/10.1007/s10905-023-09824-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-023-09824-9