Abstract
In this work, crystalline copper-zinc ferrite nanoparticles were synthesized by a simple co-precipitation method. Morphological characterization of produced samples was done using a scanning electron microscope (SEM). A transmission electron microscope (TEM) was utilised for further identification and confirmation of the particle morphology and size. Moreover, Fourier transformation infrared (FTIR) spectroscopy and X-ray diffraction (XRD) was employed to examine crystalline structure, chemical structure, and surface area respectively. Optical properties were examined by UV–Vis spectroscopy. The results indicate that the Zn0.5Cu0.5Fe2O4 nanoparticles’ crystallite size was 28.5 nm. The experiments focused on the impact of various factors, such as pH levels, initial MB concentration, and nanocatalyst dosage, on the observed photocatalytic efficiency. The photocatalytic performance of Zn0.5Cu0.5Fe2O4 nanoparticles under UV light was evaluated by decolorization of Methylene Blue (MB) azo dye. Photocatalysis degradation of 10 ppm of MB adding 15 mg of Zn0.5Cu0.5Fe2O4 nanoparticles was 94% after 135 min at room temperature and pH value of 9. Further interpretation was carried out and a proposed mechanism for the MB photodegradation by Zn0.5Cu0.5Fe2O4 nanoparticles was suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A vast class of oxides with magnetic characteristics are called ferrites [1]. Cubic spinel and magnetic ferrite (M ferrites) are the most prevalent type [2]. Spinel M ferrite in nanoparticles has particular magnetic, electrical, and catalytic qualities. Accordingly, this material is widely used in the various scientific and industrial fields due to its usefulness as a class of magnetic nanoparticles [3], such as photocatalytic activity [4], medical diagnostics, drug delivery, high-density information storage media, electrical devices, ferrofluid technology, sensors, and applications for high microwave power [5]. Their syntheses are simple and affordable methods that mainly play in the processes of mass production [6].
There have been numerous methods for creating magnetic metal ferrite nanoparticles, which include co-precipitation [7,8,9], mechanochemical [10], hydrothermal/ solvothermal [11], and sol-gel methods [12]. The co-precipitation process is a well-known technique for making ferrites [13]. This approach benefits from easy preparation, hence uncomplicated [4] and ultra-fine and homogenous powder is produced using a gentle and inexpensive chemical process [14]. On the other hand, there are many efficient techniques for treating wastewater, and water contamination is a problem that affects the entire world. The perfumed and pigmented chemical molecules identified as dyes absorb the light and produce a clear matter colour [15]. The textile industry is the sector with the highest rate of dye application, where textile dyes with very complex compounds and a variety of structural groups are used [16]. Methylene blue (MB) is a basic aromatic heterocyclic dye [17] with a molecular weight of a 319.85 g mol−1 [18, 19]. The thiazine dye, MB, is a widely used dye that has a chemical formula of C16H18N3ClS and a maximum wavelength of 663 nm. Its water solubility is classified as high, thus causing it to produce a stable solution in water at room temperature [19, 20]. MB forms a health hazard to humans if above a specific concentration rate [20]. Because MB is toxic, carcinogenic, and non-biodegradable, it possesses a great risk to human health and damaging impacts on the environment [21]. Multiple health hazards form due to the MB, including respiratory distress, metabolic and psychological issues, vision loss, and abdominal disfunctions [22]. Additionally, it results in vomiting, diarrhoea, cyanosis, indigestion, jaundice, methemoglobinemia, shock, necrotic tissues, and tachycardia, which kills premature cells in tissues and irritates the skin and eyes [23]. Zn0.5Cu0.5Fe2O4 nanoparticles are promising new materials for the photodegradation of organic contaminants. They have several advantages over commercial TiO2, including a narrow band gap, high surface area, magnetic properties, and stability. These advantages make Zn0.5Cu0.5Fe2O4 nanoparticles more efficient, reliable, and cost-effective for real-world applications.
In this work, Zn0.5Cu0.5Fe2O4 nanoparticles were synthesized through the co-precipitation method to examine their structural and optical characteristics, and its effect in the photodegradation of MB dye.
2 Materials and Methods
2.1 Chemicals
Iron (III) sulphate pentahydrate (Fe2(SO4)3·5H2O, 97%), copper (II) sulphate hexahydrate (CuSO4·6H2O, 99%), zinc (II) sulphate heptahydrate (ZnSO4·7H2O, 99%), sodium hydroxide (NaOH, ≥ 98%), and Methylene blue (MB, ≥ 97%) were from E-Merck Products. The additional reagents classified as analytical grade reagents were not purified before use.
2.2 Synthesis of Zn0.5 Cu0.5 Fe2O4
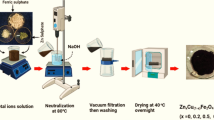
The synthesis of The Zn0.5Cu0.5Fe2O4 nanoparticles was carried out through the co-precipitation technique [24]. The synthesis plan for creating nanoparticles is depicted in Fig. 1. The stoichiometric ratios of reagent grade Fe2 (SO4)3·5H2O, CuSO4·6H2O, and ZnSO4·7H2O were firstly mixed for 30 min in 50 ml deionized water until complete solubility. Then the solution mixture was promptly added to hot 2 L NaOH solution (1 M) at 80–90 °C under vigorous stirring. Solution-based synthesis methods are sensitive to pH, which influences on the formation of the ferrite, particle size and degree of crystallinity [13]. Therefore, pH value can cause a variety of physical and chemical properties of ferrite nanoparticles. The effect of pH during formation of nanoparticles through coprecipitation route has been studied by various groups [25, 26]. Rohollah et al. [27] concluded that the crystallinity and crystallite size was increased with increasing of pH but distribution of cations not significantly change. We believe that there is a wide scope of tuning microstructure and physical properties by changing the pH value during chemical reaction of the starting solution. This aspect can be engineered for technological applications of magnetic ferrites. The obtained colloidal solution was held between 80 and 90 °C for 60 min, then left to cool down until the precipitate settled. The clear supernatant was carefully removed. This process was repeated ten times until the filtrate had a pH 7 to confirm the removal of the products Na2SO4 and the excess of NaOH [24, 28]. The samples produced were kept drying for 72 h at room temperature (20 to 25 °C).
2.3 Characterization of the Zn0.5Cu0.5Fe2O4 Nanoparticles
Different characterization methods were used for identifying the synthesized ZnxCu (1−x) Fe2O4 nanoparticles. X-ray diffractometer type (X’Pert PRO) Cu Kα radiation (λ = 1.5418 A°) was used to determine and analyse the phase present in the Cu Zn ferrite nanopowders. Scanning electron microscope (SEM) type FEI Czech at KV 25-30KV, magnification 150X, and an average working distance of 15 mm was operated for morphological and semi-quantitative elemental analysis. Additionally, a High-Resolution Transmission Electron Microscope (HRTEM) with the model number JEM-2100 from JEOL Japan was employed for detailed morphological characterization and identification of the particle size. FTIR spectra were investigated using JASCO FTIR 3600 spectrometer. UV–Vis spectra were studied by operating an Agilent Cary 60 UV–Vis spectrophotometer.
2.4 Photocatalytic Reactor
Photocatalysis was applied to degrade the MB, UV lamp in the presence of a catalyst Cu0.5Zn0.5Fe2O4. The UV reactor in use was cylindrical glass (100 ml) of 27 cm in length, 3 cm in diameter, and covered with thin film from aluminium foil. The photoreactor with a volume of 70 ml was filled with contaminated solutions.
A commercial UV–C Lamp was used as a UV light irradiation source, and PHILIPS TUV 11WG11 T5 as a high-pressure mercury lamp, 11 W with a mean wavelength of 254 nm. It is immersed in the contaminated solutions, while the photoreactor is kept at about 15 °C by a cold-water bath Fig. 2 shows the photochemical cell utilized in the photocatalytic process.
Firstly, put the pollutant MB dye and catalyst Cu0.5Zn0.5Fe2O4 into the glass cylindrical reactor and insert the UV irradiation, in the case of MB, 1 ml suspension of the MB solution was sampled using a syringe from the UV reactor at a specific time and centrifuging at 20 min. Employ a spectrophotometer to determine the absorbance at 426 nm [29]. The photodecomposition efficiency (Removal %) was estimated using the following formula [30]:
where Co is the contaminant’s starting concentration (in mg/l), and Ct represents the concentration at a given time (t).
The operational parameters of photocatalytic degradation, including starting pollutant concentration and pH, were researched.
3 Results and discussion
3.1 Characterization of Zn0.5Cu0.5Fe2O4 Nanoparticles
3.1.1 X-ray Diffraction Analysis
The XRD patterns of the produced Zn0.5Cu0.5Fe2O4 nanocatalyst are shown in Fig. 3. The X-ray diffraction patterns of the catalyst Zn0.5Cu0.5Fe2O4 exhibit the cubic phase, as indicated by the standard (JCPDS 82- 1042). There is only one distinct peak observed in the XRD pattern for each diffraction angle. The peak is clearly visible at about 2θ values of 29.5°, 35.2°, 42.5°, 36.5°, 53.1°, 56.3°, and 62° [31]. Therefore, the principal diffraction peak of cubic spinel ferrite occurs at the (311) plane and corresponds to a 2θ value of around 35.2o. The average size of the crystallites was determined by analysing the broadening of the diffraction peak. The calculation was performed using the Scherrer equation, as documented in references [32, 33].
where ß is the perceived diffraction peak’s breadth at half its maximum intensity, K is a shape factor, which has an approximate value of 0.9 and is equal to 0.15406, the wavelength of CuK’s X-rays [29]. The Zn0.5Cu0.5Fe2O4 nanoparticles’ crystallite size was 28.5 nm at the (311) plane, which was the strongest peak. It is observed that the sample Zn1− x Cux Fe2 O4 crystallized in cubic structure and the lattice parameter is found to be 8.355 Å, which is less than the values reported for bulk compounds. This is mainly because of the replacement of smaller Cu2+ ions (0.72 Å) by larger Zn2+ ions (0.74 Å) [34, 35]. In addition, as the ionic radius of Zn2+ is larger than the ionic radius of Cu2+, the Zn2+ substitution leads to larger expansion of the lattice. Consequently, the lattice parameter increases more when compared to the Cu2+ substitution in the synthesized particles. Since ionic radius of Fe2+ (0.74 Å) ion is larger than Fe3+ ion (0.64 Å), the lattice constant increases [36].
3.1.2 Morphological Analysis of Zn0.5Cu0.5Fe2O4Nanoparticles
In Fig. 4a, the SEM image of Zn0.5Cu0.5Fe2O4 nanocrystals shows that the shape of the synthesized nanoparticle resembles a sphere, consistently clustered, and the grain size ranging from 25 to 35 nm. The EDAX analysis of Zn0.5Cu0.5Fe2O4 is shown in Fig. 5. It explains the relative presence of Zn, Cu, Fe, and O. TEM was employed successfully to acquire additional facts about the morphology study. As shown in Fig. 4b, obvious large clusters consisting of smaller particles are apparent. The observed particle size during the SEM imaging was not representative enough for the sample. Details in the SEM images may be lost due to the surface charging of samples during interaction with an electron beam. A charge builds up that cannot be released from the surface, especially in the absence of the atmosphere, as the electron beam is allowed to raster over the surface of insulating metal oxides. These phenomena lead to losing SEM magnification.
3.1.3 FTIR Analysis
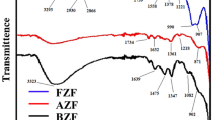
The produced Zn0.5Cu0.5Fe2O4 ferrite nanoparticles’ FT-IR spectrum shown in Fig. 6 was executed with a frequency ranging from 500 to 4000 cm−1. In general, the obtained spectra may be divided into two main regions; the first one represented is the fingerprint region (400–1800 cm−1) and the other is the lattice water H-O-H stretching band (3200–3600 cm−1) [37, 38]. Sharp absorption bands between 550 and 700 cm−1 are credited to the tetrahedral groups’ inherent vibrations [31]. The absorption band at 1100 cm−1 may be assigned to the two metal-oxygen stretching modes M=O. those with only one terminal oxygen atom absorb [38]. The peak at 1631 cm−1 is due to the O–H bending vibration of adsorbed water molecules from the adsorption of moisture on the surface of the nanoparticle, and at 3400 cm−1 due to the stretching mode of the adsorbed O–H group’s O–H group.
3.1.4 UV–Visible Absorption, Band Gap and Photoluminescence (PL) Analysis for Synthesized Zn0.5Cu0.5Fe2O4 NPs
Optical properties of prepared Zn0.5Cu0.5Fe2O4 nanoparticles was analyzed in the range of 200–800 nm. Optical absorption was used to evaluate the energy gap of the nanostructures shown in Fig. 7a. As can be seen from Fig. 7a, The UV–visible spectrum demonstrates that Zn0.5Cu0.5Fe2O4 NPs possesses a high absorptivity in the 300–500 nm wavelength region because of the part the hybridized Fe–d orbital plays [39]. The band gap energy was determined from the absorption spectra using Tauc relation [40], as shown in the inset of Fig. 7a, and found to be around 1.8 eV. It should be mentioned here that with higher band gap energy, the recombination rate of electrons and hole pairs are retarded, and photocatalytic properties are enhanced [15]. Figure 7b shows the photoluminescence (PL) spectra of Zn0.5Cu0.5Fe2O4 nanoparticles taken at an excitation wavelength of 365 nm, The photoluminescence spectrum shows two major peaks, one at 698 nm assigned to the bandgap excitons and the other at ~ 780 nm attributed to the surface-related emission (or more delicately, both surface and interface) [41].
3.2 Photocatalytic Potential of Zn0.5Cu0.5Fe2O4 Nanoparticles on MB
The photocatalytic potential of the prepared Zn0.5Cu0.5Fe2O4 nanoparticles was examined concerning MB removal as a contaminant under UV lamp radiation. Methylene blue is a commonly used thiazine dye with highly carcinogenic properties that has been manufactured and used in various industries for many purposes, including the colouring of paper, the dyeing of cotton, wool, and textiles, the colouring of hair, and as a medication [42]. Methylthioninium chloride or 3,7-bis(dimethylamino)phenothiazin-5-ium are its alternative names [18]. Figure 8 displays the MB structural formula [43].
Figure 9 shows the stability of MB contaminant at a concentration 10 ppm in photolysis of MB, adsorption in the dark, and photocatalysis under UV for 135 min. A UV–Vis DR5000 spectrophotometer measured the amount of MB dye concentration at a wavelength of 664 nm [44]. It was clear that there was no appreciable degradation of MB in water, which was predicted and validated with the MB concentration used. Furthermore, the photolysis of MB was insignificant because the OH· and H· radicals it produced were ineffective in UV-oxidation processes in the absence of an appropriate catalyst [45].
Different UV irradiation protocols are illustrated in the references. Some recommend delayed UV irradiation by 30 min or more to eliminate the adsorption effect [46]. On the other hand, many authors recommend the combination of UV irradiation and adsorption without any delay [47]. A comparative test was employed to determine the significance of delayed irradiation or starting the UV irradiation from zero time. Other experiments were carried out with a fixed catalyst concentration (10 mg) and MB concentration (10 ppm). Further experimental parameters were likewise fixed, such as pH 9 and temperature 25 °C.
3.2.1 Effect of pH
The concentration of hydrogen ions in the solution was a key relying factors for the 90-minute removal search under predetermined experimental situations (10 mg of nanocomposite produced, 50 ml of 10 mg/l, MB solution at temperature = 25 °C), where the result of the beginning values of solution pH was examined. Figure 10a exhibits a plot of the variance in MB removal (%) over time at various pH values (3.0, 5.0, 7.0, and 9.0). At pH 9.0, the greatest MB Removal in equilibrium was noted.
10 mg (Zn0.5Cu0.5Fe2O4NPs) 50 mL of 0.01 M NaCl solution was added to conclude the point of zero charge (PZC) of the Zn0.5Cu0.5Fe2O4 NPs. The pH of the solutions was changed to 2, 4, 6, 8, 10, and 12 using HCl or NaOH, and at 200 rpm for 48 h the samples agitated. After (Zn0.5Cu0.5Fe2O4NPs) were separated magnetically, the solutions’ pH levels were assessed.
The pH of the point of zero charge of Zn0.5Cu0.5Fe2O4 was established by a plot that compares the end and beginning pH levels, as seen in Fig. 10b. The pH of the PZC was found to be 6.74, as shown in Fig. 10b, where the pH readings at the beginning and end do not differ significantly. It shows that the surface charge of the Zn0.5Cu0.5Fe2O4 photocatalyst is positive and negative, respectively, when pH < PZC and pH > PZC. Once the pH of the solution matches the pH of the PZC, the photocatalyst surface charge is neutral and the electrostatic interaction between the photocatalyst surface and MB ions is insignificant [48]. At pH 9.0, there is maximal photocatalytic degradation of MB, which is seen in Fig. 10a, and this result was explained by the pH of the PZC value, which indicated that the pH of the PZC regarding Zn0.5Cu0.5Fe2O4 was 6.74. Thus, the Zn0.5Cu0.5Fe2O4 NPs’ net surface charge was positive, which attracts MB’s negative charge and enhances MB’s photocatalytic destruction. At pH = 5.0, the photocatalytic degradation of MB began to decline. This is because the Zn0.5Cu0.5Fe2O4 NPs’ net surface charges are positive at this pH, and the positive surface charges of MB and the Zn0.5Cu0.5Fe2O4 nanocomposite repel one another.
3.2.2 Effect of the Initial Concentration
Because the beginning concentration of the MB was crucial to the elimination procedure, the effect of MB ionic strength was assessed by adjusting it while leaving the rest of the reaction conditions controlled. Figure 11a shows the variations in removal percentage as a function of contact time for the varied initial concentrations of the MB (5.0, 10.0, and 15.0 mg/l). These results demonstrate that even at high initial concentrations, MB may be effectively removed when a synthesized Zn0.5Cu0.5Fe2O4 nanocatalyst is also present under UV light irradiation. An inverse correlation is present between MB concentration and the rate of deterioration.
3.2.3 Effect of the Nanocomposite Dose on Degradation Efficiency
Figure 11b demonstrates that by adjusting the amount of the produced photocatalyst between 5 and 20 mg against a given concentration of MB (10 mg/l), it is possible to examine how a nanocomposite dose may influence the removal effectiveness of MB below UV light. The results indicated that as the photocatalyst dose increased from 5 to 15 mg, the removal efficiency grew. The maximum photodegradation of MB using 15 mg/l of photocatalyst was 94% after 135 min at ambient temperature 25oC and pH value of 9. An increase in the photocatalyst’s accessible active area or active sites relative to the volume of the MB solution may explain the observed improvement in removal efficiency with increasing the quantity of photocatalyst in the reaction [49].
3.3 Kinetic Studies
The rate of MB degradation can be calculated by the following equation:
where the appropriate beginning and remaining concentrations of MB are (Ct and Co), (t) is the removal period, and k is the elimination rate constant. Figure 12a shows the relationship between (− ln Ct/Co) and time.
The outcomes show that the kinetics of the reduction process obeys pseudo-first-order rate laws. As seen in Fig. 12b, the pseudo-first-order rate constant decreases with the increasing catalyst dosage. This emphasis on rate constants is consistent with the literature presented [50].
a For a linear fit, pseudo-first-order model data are reported in kinetic form for MB degradation under UV irradiation with beginning concentrations of 10 ppm MB, 50 mL of 5, 10, and 15 mg catalyst doses. b Describes the link between the initial MB concentration and the apparent pseudo-first order rate constants
3.3.1 Mechanism of Photocatalysis of MB
As reported previously [51,52,53], the possible photocatalytic activity mechanism is described as follows. Explicit oxidation by positive valence band holes, explicit reduction by conduction band electrons, and hydroxyl radical assault are three photodegradation processes that are impacted by pH changes. Photocatalytic degradation will occur in the presence of the used light-activated catalyst, Zn0.5Cu0.5Fe2O4, due to the electron-hole pairs induced by the UV on its surface. The reactive MB reacts with the −OH groups to produce hydroxyl radicals or is oxidized by the holes’ oxidative potential to produce a breakdown product [51]. The following table lists the reactions between MB and the employed photocatalyst. (Eqs. 4–7).
Figure 13 depicts the proposed method of interaction between the MB and the prepared nanocomposite. Once the Zn0.5Cu0.5Fe2O4 NPs have been activated by UV light, the redox process begins. The resultant free radicals, including OH and O2, will break down the dye into smaller organic molecules [54]. There are no current studies on the degradation of MB that have been reported, thus additional study incorporating high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) is crucial for the advancement in the studies of MB degradation products.
4 Conclusion
Nanocrystalline Zn0.5Cu0.5Fe2O4 nanocatalyst was successfully prepared via a co-precipitation technique and characterized using structural and optical tools. The photocatalytic efficiency of the prepared Zn0.5Cu0.5Fe2O4 nanocatalyst was tested against MB dye. Moreover, numerous parameters that have an impact on the efficiency of removal potential such as (pH on degradation of MB, MB initial concentration, and photocatalyst dose) have been investigated. Zn0.5Cu0.5Fe2O4NPs achieved a significant photodegradation potential for Methylene Blue in aqueous solutions. The extreme photocatalytic effectiveness successfully got rid of around 94% of 10 ppm MB after 135 min with a 15 mg Zn0.5Cu0.5Fe2O4NPs at pH9. The synthesized Zn0.5Cu0.5Fe2O4 nanocatalyst is promising as a potential application in wastewater treatment.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
M. Amiri, M. Salavati-Niasari, A. Akbari, Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 265, 29–44 (2019)
A. Hakeem et al., Magnetic, dielectric and structural properties of spinel ferrites synthesized by sol-gel method. J. Mater. Res. Technol. 11, 158–169 (2021)
V.F. Cardoso et al., Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 7(5), 1700845 (2018)
P.A. Vinosha et al., Study on cobalt ferrite nanoparticles synthesized by co-precipitation technique for photo-fenton application mechanics. Mater. Sci. Eng. J. (2017)
F. Sharifianjazi et al., Magnetic CoFe2O4 nanoparticles doped with metal ions: a review. Ceram. Int. 46(11), 18391–18412 (2020)
P.G. Jamkhande et al., Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53, 101174 (2019)
M.S.A. Darwish et al., Synthesis of magnetic ferrite nanoparticles with high hyperthermia performance via a controlled co-precipitation method. Nanomaterials. 9(8), 1176 (2019)
A. Boudjemaa et al., M-substituted (M = Co, Ni and Cu) zinc ferrite photo-catalysts for hydrogen production by water photo-reduction. Int. J. Hydrog. Energy. 41(26), 11108–11118 (2016)
A.M. El-Khawaga et al., Synthesis and applicability of reduced graphene oxide/porphyrin nanocomposite as photocatalyst for waste water treatment and medical applications. Sci. Rep. 12(1), 17075 (2022)
H. Yang et al., Synthesis of ZnFe2O4 nanocrystallites by mechanochemical reaction. J. Phys. Chem. Solids. 65(7), 1329–1332 (2004)
H. Xu et al., A comparative study of nanoparticles and nanospheres ZnFe2O4 as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 7(9), 7976–7983 (2012)
N. Rajput, Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 7(6), 1806 (2015)
P. Sivagurunathan, S.R. Gibin, Preparation and characterization of nanosized cobalt ferrite particles by co-precipitation method with citrate as chelating agent. J. Mater. Sci.: Mater. Electron. 27, 8891–8898 (2016)
Z. Zhang et al., Synthesis and characterization of nickel ferrite nanoparticles via planetary ball milling assisted solid-state reaction. Ceram. Int. 41(3), 4523–4530 (2015)
A.M. El-Khawaga et al., Promising photocatalytic and antimicrobial activity of novel capsaicin coated cobalt ferrite nanocatalyst. Sci. Rep. 13(1), 5353 (2023)
O.H. Abuzeyad et al., An evaluation of the improved catalytic performance of rGO/GO-hybrid-nanomaterials in photocatalytic degradation and antibacterial activity processes for wastewater treatment: a review. J. Mol. Struct. 1288, 135787 (2023)
L.A. Attia, Modified bentonite as adsorbent material for the removal of the basic dye methylene blue from aqueous solutions. J. Part. Sci. Technol. 7(1), 23–31 (2021)
I. Khan et al., Review on methylene blue: its properties, uses, toxicity and photodegradation. Water. 14(2), 242 (2022)
N. Marzban et al., Intelligent modeling and experimental study on methylene blue adsorption by sodium alginate-kaolin beads. Int. J. Biol. Macromol. 186, 79–91 (2021)
R.D.C. Soltani et al., The application of ZnO/SiO2 nanocomposite for the photocatalytic degradation of a textile dye in aqueous solutions in comparison with pure ZnO nanoparticles. Desalin. Water Treat. 56(9), 2551–2558 (2015). https://doi.org/10.1080/19443994.2014.964781
S. Zhang et al., Non-biodegradable microplastics in soils: a brief review and challenge. J. Hazard. Mater. 409, 124525 (2021)
M. Oz et al., Cellular and molecular actions of methylene blue in the nervous system. Med. Res. Rev. 31(1), 93–117 (2011)
E.C.Z. Qing, F.A.X. Ying, S.C.N.Y. Rong, Biochar Derived from Chopsticks as an Effective Biosorbent for Pollutant Removal from Wastewater
A. Jain et al., Study of Zn-Cu ferrite nanoparticles for LPG sensing The Scientific World Journal, 2013. 2013
S. Rani, G.D. Varma, Superparamagnetism and metamagnetic transition in Fe3O4 nanoparticles synthesized via co-precipitation method at different pH. Phys. B: Condens. Matter. 472, 66–77 (2015)
M.O. Besenhard et al., Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: new insights and continuous production via flow chemistry. Chem. Eng. J. 399, 125740 (2020)
R. Safi et al., The role of pH on the particle size and magnetic consequence of cobalt ferrite. J. Magn. Magn. Mater. 396, 288–294 (2015)
A.I. Ahmed et al., Structural and optical properties of Mg1-x Znx Fe2 O4 nano-ferrites synthesized using co-precipitation method. Adv. Nanopart. 4(02), 45 (2015)
R. Solomon et al., Enhanced photocatalytic degradation of azo dyes using nano Fe3O4. J. Iran. Chem. Soc. 9, 101–109 (2012)
E.S. Aziman, A.F. Ismail, Removal of Thorium from Industrial Waste via Electrosorption Technique. IOP Publishing
H. Nayak, Effect of catalytic activities of mixed nano ferrites of zinc and copper on decomposition kinetics of lanthanum oxalate hydrate. Trans. Nonferrous Met. Soc. China. 26(3), 767–774 (2016)
W. Shi et al., Magnetic nano-sized cadmium ferrite as an efficient catalyst for the degradation of Congo red in the presence of microwave irradiation. RSC Adv. 5(63), 51027–51034 (2015)
S.M.A. Ridha, X-ray studies and electrical properties of the zinc-substituted copper nanoferrite synthesized by sol-gel method. Int. J. Compos. Mater. 5(6), 195–201 (2015)
X. Tan et al., The effect of Cu content on the structure of Ni1–xCuxFe2O4 spinels. Mater. Res. Bull. 44(12), 2160–2168 (2009)
M. Sundararajan et al., Enhanced visible light photocatalytic degradation of rhodamine B using Ni1-xCaxFe2O4 (0 ≤ x ≤ 0.5) nanoparticles: performance, kinetics and mechanism. Mater. Res. Bull. 154, 111911 (2022)
A.R. Denton, N.W. Ashcroft, Vegard’s law. Phys. Rev. A 43(6), 3161–3164 (1991)
M.S. Giri et al., Influence of Aluminium ion substitution on Structural and Magnetic Properties of nano-structured BaFe12O19 Powder
G. Socrates (2001) Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Wiley
R. Dom et al., Synthesis of solar active nanocrystalline ferrite, MFe2O4 (M: ca, zn, mg) photocatalyst by microwave irradiation. Solid State Commun. 151(6), 470–473 (2011)
J. Tauc, J. Tauc,(1974) Amorphous and liquid semiconductor, Plenium Press New York, NY, USA,
C.S. Stan, M.S. Secula, D. Sibiescu, Highly luminescent polystyrene embedded CdSe quantum dots obtained through a modified colloidal synthesis route. Electron. Mater. Lett. 8, 275–281 (2012)
T. Cwalinski et al., Methylene blue—current knowledge, fluorescent properties, and its future use. J. Clin. Med. 9(11), 3538 (2020)
P. Temeepresertkij, M. Iwaoka, S. Iwamori, Molecular interactions between methylene blue and sodium alginate studied by molecular orbital calculations. Molecules. 26(22), 7029 (2021)
N.X. Sang et al., Crystalline deformation and photoluminescence of titanium dioxide nanotubes during in situ hybridization with graphene: an example of the heterogeneous photocatalyst. Superlattices Microstruct. 121, 9–15 (2018)
S.M. Anisuzzaman et al., Current trends in the utilization of photolysis and photocatalysis treatment processes for the remediation of dye wastewater: a short review. ChemEngineering. 6(4), 58 (2022)
M. Bagheri, A.R. Mahjoub, B. Mehri, Enhanced photocatalytic degradation of Congo red by solvothermally synthesized CuInSe 2–ZnO nanocomposites. RSC Adv. 4(42), 21757–21764 (2014)
M.F. Attallah, I.M. Ahmed, M.M. Hamed, Treatment of industrial wastewater containing Congo Red and Naphthol Green B using low-cost adsorbent. Environ. Sci. Pollut. Res. 20, 1106–1116 (2013)
F. Azeez et al., The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 8(1), 7104 (2018)
N.P. Ferraz et al., CeO 2–Nb 2 O5 photocatalysts for degradation of organic pollutants in water. Rare Met. 39, 230–240 (2020)
A. Ara et al., Synthesis, characterization, and Solar Photo-Activation of Chitosan-Modified Nickel Magnetite Bio-composite for Degradation of recalcitrant Organic pollutants in Water. Catalysts. 12(9), 983 (2022)
K.C. Bedin et al., KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 286, 476–484 (2016)
M. Sundararajan et al., Photocatalytic degradation of rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram. Int. 43(1), 540–548 (2017)
S. Kanithan et al., Enhanced optical, magnetic, and photocatalytic activity of Mg2 + substituted NiFe2O4 spinel nanoparticles. J. Mol. Struct. 1265, 133289 (2022)
M. Sundararajan et al., Visible light driven photocatalytic degradation of rhodamine B using mg doped cobalt ferrite spinel nanoparticles synthesized by microwave combustion method. J. Phys. Chem. Solids. 108, 61–75 (2017)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: OHA, AMEK, HT, MG and MAE; Formal analysis: OHA, AMEK, HT, MG and MAE; Writing—Original draft preparation: AOHA, AMEK, HT, MG and MAE; Writing—Review and editing: OHA, AMEK, HT, MG and MAE. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abuzeyad, O.H., El-Khawaga, A.M., Tantawy, H. et al. Photocatalytic Degradation of Methylene Blue Dye by Promising Zinc Copper Ferrite Nanoparticles for Wastewater Treatment. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03006-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03006-6