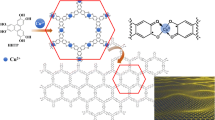
Abstract
Multilayered coordination nanosheets made of copper ions and hexaaminobenzene (CuHAB) were synthesized in a liquid–liquid interfacial reaction in the presence of a base and an oxidizing agent. X-ray diffraction studies revealed the suitability of an oxidizing agent with a low oxidation potential for the synthesis of highly crystalline CuHAB. The base concentration affects the chemical and crystal structures of CuHAB. The nanosheets synthesized at low base concentrations exhibit humidity-responsive conductivity due to the ligating amino group. Meanwhile, the nanosheets prepared at high base concentrations have different stacking structures from the CuHABs formed at low base concentrations and show greater conductivity. These results indicate that the liquid–liquid interfacial synthesis with an appropriate oxidizing agent and quantitative addition of base can modulate chemical and stacking structures of coordination nanosheets composed of hexaaminobenzene ligand and their functionalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coordination nanosheets, which are a new class of two-dimensional materials constructed by hybridizing transition metal ions and organic bridging ligands, have attracted much attention recently. Their highly customizable chemical structures derived from a wide variety of combinations of metal ions and ligands and the ease of synthetic procedures based on coordination reactions allow development of materials with various chemical and physical properties [1,2,3,4,5]. Bis(diimino)metal coordination nanosheets (MHABs), composed of metal ions and hexaaminobenzene (HAB) ligands, have electronic conductivity derived from a delocalized π-conjugation system in planar, porous structures, and redox activities of metal complex units. They have proven useful in applications such as electrode materials for energy storage [6,7,8,9], supercapacitors [10, 11], electrocatalysts for methanol oxidation, hydrogen evolution, and oxygen evolution reactions [12, 13], and for chemiresistive gas sensors [14,15,16,17]. Three synthetic methods for MHABs, a one-phase solution reaction [15,16,17], a gas–liquid interfacial reaction [15, 18], and electrochemical oxidation [18], have been reported. In forming bis(diimino)metal complexes, oxidation of HAB ligand is essential. Hence, in conventional synthesis, dioxygen (air) is added as an oxidizing agent to a gas phase of the reaction vessel to form a film at the gas–liquid interface, or electrochemical oxidation is used to form a polymerized film on a working electrode. However, air-oxidation is not suitable for quantitative oxidation, and it is not easy to obtain a free-standing film using the electrochemical method.
In the present work, we employ liquid–liquid interfacial synthesis with quantitative addition of an oxidizing agent and base in order to synthesize multi-layered, copper-HAB coordination nanosheets (CuHAB-X, X = 1, 10, or 100, where X denotes the concentration of Na2CO3 in aqueous solution) as free-standing films. Characterization by microscopic, spectroscopic, and diffraction analyses indicates that morphologies, chemical structures, and periodic structures of CuHAB-X films depend on the base concentration. CuHAB-10 and CuHAB-100 films have only imino structure, while the CuHAB-1 film contains both amino and imino structures. CuHAB-10 and CuHAB-100 develop a slipped stacking structure and exhibit humidity-independent conductivity due to the lack of amino groups in the structures. In contrast, amino groups in CuHAB-1, which can form hydrogen bonds between interlayer or water molecules, develop eclipsed-like stacking and humidity responsive conductivity. Humidity-responsive conducting properties of amorphous MHABs (M = Ni, Cu, Co, and Fe) introduced by structural defects were reported by Ding and co-workers [14]. This study demonstrates another origin of humidity-response conductivity of CuHAB, based on the coordination structure containing the amino groups.
2 Experimental
2.1 Materials
Bis(2,2,6,6-tetramethyl-3,5-heptanedionato)copper (Cu(TMHD)2), Na2CO3, and 2,5-di-tert-butyl-1,4-benzoquinone (tBu2BQ) were purchased from commercial sources and used without further purification. Hexaaminobenzene trihydrochloride (HAB∙3HCl) was synthesized according to the literature [19, 20] or purchased from Toronto Research Chemicals. Water was purified by an Autopure WD500 (Yamato Scientific). All HPLC-grade organic solvents for synthesis were purchased from KANTO CHEMICAL.
2.2 Equipment
AFM topography images were obtained using an Agilent Technologies 5500 scanning probe microscope with an NCH silicon cantilever (NanoWorld) in the tapping mode. TEM images were recorded at 200 kV of the accelerating voltage using a JEOL JEM-2100F. FT-IR spectra of KBr-pelletized CuHAB films were recorded using an FT/IR-6100 (JASCO). Before the IR measurements, the samples were dried under vacuum for 30 min in the sample chamber of the spectrometer. X-ray photoelectron spectra were collected using PHI 5000 VersaProbe (ULVAC-PHI) with a monochromatic Al Kα X-ray source. The spectra were standardized using a C 1s peak of the adventitious carbon at 284.6 eV. Powder X-ray diffraction (PXRD) and grazing-incidence X-ray scattering (GIXS) measurements were conducted using synchrotron radiation at Beamline BL44B2 (λ = 0.8 Å) [21] and BL05XU (λ = 1 Å) in Super Photon ring-8 (SPring-8), respectively. Samples for PXRD measurement were prepared by collecting CuHAB films by centrifugation after the liquid–liquid interfacial synthesis (see below), then dried under vacuum. Samples for GIXS measurements were prepared by drop-casting a flake in an ethanol dispersion obtained after the liquid–liquid interfacial reaction on a silicon substrate.
2.3 Conductivity Measurement
An ethanol dispersion of CuHAB was drop-casted on a gold interdigitate electrode patterned on a glass substrate (GMT-AU10/5, GEOMATEC) which has a 10-μm width of Au electrodes with a 5-μm interval. The electrode was connected to an HZ-Pro multi-electrochemical measurement system (HOKUTO DENKO) and placed in an incubator to keep the measurement temperature at 25°C. The conductivity measurement was conducted using the two-probe method with the humidity of ambient, 10% RH, and 75% RH. The humidity was controlled by an AHCU-2 humidity controller (KITZ MICRO FILTER CORPORATION).
2.4 Liquid–Liquid Interfacial Synthesis of CuHAB
Under an argon atmosphere, an aqueous solution of HAB∙3HCl (1 mM, 2.8 mg/10 mL) and Na2CO3 (1, 10, or 100 mM, 1.1, 10.6, or 106 mg/10 mL, respectively), and a dichloromethane solution of Cu(TMHD)2 (2 mM, 8.6 mg/10 mL) and tBu2BQ (3 mM, 6.6 mg/10 mL) was prepared. A 5 mL of the dichloromethane solution was added in a 20 mL vial, followed by layering with pure water (5 mL) to form a liquid–liquid interface. The aqueous solution (5 mL) was then gently added to the water phase. The reaction container was left under an argon atmosphere without disturbance. After 1 day, CuHAB was formed at the interface as a black film. After the reaction, the upper aqueous phase was washed with water and substituted with ethanol. Then, the bottom organic layer was removed to obtain an ethanol dispersion of CuHAB film. The samples were named CuHAB-X, where X denotes the concentration of Na2CO3 aqueous solution. In addition, CuHAB-NB was synthesized by the same procedure in the absence of Na2CO3 and used as a reference sample.
3 Results
3.1 Optimization of Oxidizing Agent
In the MHAB formation process, the strength of the oxidizing agent is a factor affecting the complexation reaction rate, and slow crystal growth will be suitable for the formation of highly crystalline samples. We surveyed an appropriate oxidizing agent for crystalline CuHABs using 1,4-benzoquinone derivatives because their oxidizing potentials can be easily modified by the substituent groups. Generally, the substituent of hydrogen atoms in 2, 3, 5, and/or 6 positions with electron-withdrawing groups such as halogen atoms, nitro, and carboxyl group make the redox potential positive giving strong oxidizing ability while the substituent with electron-donating groups such as methoxy, methyl, and tert-butyl groups give negative redox potentials showing weak oxidizing ability [22]. CuHABs were synthesized at ethyl acetate/H2O and H2O/dichloromethane liquid–liquid interface with chloranil, 2,5-dichloro-1,4-benzoquinone, 2,5-dimethyl-1,4-benzoquinone, 2,5-dimethoxy-1,4-benzoquinone, and 2,5-di-tert-butyl-1,4-benzoquinone (tBu2BQ), then their crystallinities were evaluated by powder X-ray diffraction or grazing-incidence X-ray scattering methods (See Supporting Information and Fig. S1 for detail). The series of diffraction patterns revealed that CuHABs synthesized with strong oxidizing agents such as chloranil, 2,5-dichloro-1,4-benzoquinone, and 2,5-dimethyl-1,4-benzoquinone exhibited broad diffraction peaks, suggesting low crystallinities. On the other hand, CuHABs prepared using 2,5-dimethoxy-1,4-benzoquinone, and tBu2BQ as weak oxidizing agents showed obvious peaks derived from higher crystallinity. Hence, an oxidizing agent with low oxidation potential is suitable for the synthesis of highly crystalline CuHAB films. Although 2,5-dimethoxy-1,4-benzoquinone and tBu2BQ give CuHABs with comparable crystallinities, the latter has higher solubility in various organic solvents. Hence, tBu2BQ was selected as the oxidizing agent for further experiments.
3.2 Microscopic Characterization
A liquid–liquid interfacial reaction between a dichloromethane solution of Cu(TMHD)2 and tBu2BQ and an aqueous solution of HAB∙3HCl and Na2CO3 forms CuHAB-X as a bluish-black film at the interface (Fig. 1). The resulting CuHAB sheets were characterized using atomic force microscopy (AFM), transmission electron microscopy (TEM), IR spectroscopy, X-ray photoelectron spectroscopy (XPS), and synchrotron powder X-ray diffraction (PXRD).
AFM analysis revealed that CuHAB-1 has a thickness of approximately 0.3 μm, whereas CuHAB-10 and CuHAB-100 are thicker films, with thicknesses of 1.5 − 4 μm (Fig. 2). A possible reason for the variable thickness is that the high Na2CO3 concentration accelerates the coordination reaction at the liquid–liquid interface, forming thick CuHAB films. CuHAB-100 exhibits rough surface morphology compared with those of CuHAB-1 and CuHAB-10.
Detailed morphology and periodicity of CuHAB-X films were achieved with TEM. CuHAB-1 is composed of a crystalline phase with domains 50-nm-diameter, as well as an amorphous phase, and CuHAB-10 displays the largest crystalline domains with a diameter larger than 100 nm (Fig. S2). These observed domain sizes are larger than MHABs synthesized with the conventional one-phase reaction method, which gives a crystalline domain with a diameter of 15–30 nm [6, 8]. A high-resolution image of the crystalline phase and its fast Fourier transform (FFT) image indicate that CuHAB-1 and CuHAB-10 have hexagonal lattices with a periodicity of 1.3 nm, consistent with previous reports (Fig. 2). Hence, the liquid–liquid interfacial reaction is useful for producing crystalline CuHAB films. In contrast, CuHAB-100 is formed as an aggregate of small crystalline CuHAB domains with diameters of 15 nm (Fig. 2). Formation of larger crystal domains might be prevented due to the faster coordination reaction of deprotonated HAB in a solution with a higher Na2CO3 concentration. This may explain the rough surface structure of CuHAB-100 observed under AFM. FFT images show that the crystalline phase of CuHAB-100 also has a hexagonal framework.
3.3 IR and Raman Spectroscopy
IR and Raman spectroscopy measurements were employed to evaluate chemical structures in the CuHAB films (Fig. 3). All samples show peaks around 3300, 1410 and 1200 cm−1 attributed to N–H stretching, aromatic C=C stretching, and C–N stretching, respectively in the IR spectra. These peaks are consistent with previous reports of CuHAB IR spectra. A broad peak at 3450 cm−1 derives from H2O contained in the sample because the peak disappearance was observed after the overnight drying under vacuum of the KBr-pelletized sample at 120 °C while the peak appeared again after the overnight exposure of the sample to air with RH75% (Fig. S3). Insignificant changes in the other peaks derived from CuHABs suggest the sample durability under high-temperature and high-humid conditions. Focusing on the band of N–H stretching, CuHAB-1 has a shoulder peak around 3250 cm−1 while the peak intensities of the other samples near this wavenumber are weaker than CuHAB-1 (Fig. 3b). CuHAB-NB prepared in a base-absent condition also showed a corresponding shoulder peak around 3300 cm−1 (Fig. S4). Furthermore, an IR spectrum of an intermediate of CuHAB obtained in the absence of a base and air (oxidizing agent) gives a strong peak around 3200 cm−1 attributed to N–H stretching in the amine group, also suggesting the existence of amine structures in CuHAB-1 [10]. Hence, the shoulder peak observed in CuHAB-1 suggests that CuHAB-1 contains another N–H stretching mode in addition to N–H stretching derived from the imine structure. The most conceivable structure is the amino group, which is not deprotonated due to the low base concentration during synthesis. In addition, no significant peaks derived from C=O stretching (ca. 1650 cm−1) and C-H stretching vibrations (2800–3000 cm−1) indicated that CuHAB films do not encapsulate tBu2BQ and 2,2,6,6-tetramethyl-3,5-heptanedione which is the ligand of Cu(TMHD)2.
The Raman spectra of CuHAB-X films exhibited peaks at 450 cm−1 and in the regions of 1350–1700 cm−1 deriving from Cu–N stretching and the aromatic ring stretching, respectively, which are in good agreement with the Raman spectra of CuHAB powder samples synthesized by one-phase reactions (Fig. S5) [9, 15].
3.4 Elemental and Chemical State Analysis by XPS
Elemental analysis and evaluation of the chemical state of CuHAB-X employed XPS measurements (Figs. 4 and S6–S8). CuHAB-1 shows a strong Cu 2p3/2 peak at 934 eV with a shoulder peak at lower binding energy (932 eV), whereas both CuHAB-10 and CuHAB-100 give two obvious peaks of Cu 2p3/2 at 934 and 932 eV. These two peaks correspond to the Cu(II) and Cu(I) states of copper ions in CuHAB films. Peak fitting applied to the Cu 2p3/2 peak reveals that the ratio of Cu(II):Cu(I) is 2:8 for CuHAB-1 and 3:7 for both CuHAB-10 and CuHAB-100 (Fig. S7). These results indicate that copper ions in CuHAB assume mixed valence states and that the Cu(I) ratio decreases with increasing Na2CO3 concentration. Peaks observed at 952 and 954 eV are assignable to Cu 2p1/2 peaks of Cu(I) and Cu(II), respectively, and broader peaks around 945 and 962 eV are satellite peaks derived from Cu(II) (Fig. S6). The N 1 s region gives detailed information about the chemical state of nitrogen atoms in CuHAB. The observed peaks can be reproduced by fitting three peaks for the negatively charged nitrogen species such as C−NH− (397.5 eV), the charge-neutral nitrogen species such as C−NH2 and C=NH (399.0 eV), and the nitrogen atom not coordinated to a copper center (Free N, 401 eV). Based on the peak area calculation, CuHAB-1 has a higher abundance of neutral nitrogen species (43%) compared to other CuHABs (ca. 23%) because of the existence of amino groups as implied in IR spectra (Fig. S7). This is because complete deprotonation was not achieved in a low base concentration; hence, the amino group of HAB remains intact after the coordination reaction. CuHAB-NB also showed a high existence ratio of neutral nitrogen species (39%) (Fig. S8).
3.5 Crystal Structures of CuHABs
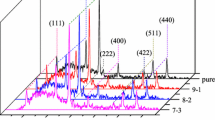
In order to determine the detailed periodic structure of CuHAB films, PXRD measurements were conducted (Fig. 5). The resulting diffraction patterns indicate that CuHAB-1 has a periodic structure different from those of CuHAB-10 and CuHAB-100. CuHAB-1 exhibited diffraction peaks at 4.0°, 8.0°, 10.5°, 11.9°, and 14.3°. This is similar to the reported pattern of bis(diimino)copper complex frameworks synthesized by the previous O2-oxidation method, which adopts the eclipsed (AA) stacking in the P6/mmm space group [18]. However, according to IR and XPS results, CuHAB-1 contains N atoms forming imino and amino structures, whereas reported structures assume that all N atoms take the imino rather than the amino structure. It is estimated that the copper complex unit in CuHAB-1 has bis(iminoaminato)copper structure, which contains two imino-type N atoms and two amino-type N atoms coordinated to the Cu center. In contrast, if CuHAB-1 assumed the eclipsed stacking structure, the interlayer distance is expected to be larger because of the steric hindrance of the hydrogen atoms of the amino group in the out-of-plane position. Alternate stacking of amine-type and imine-type N atoms of the copper complex units is expected to reduce this steric hindrance. An AA’ stacking model is given as a structure of CuHAB-1 with the P31c space group and cell parameters of a = 13.2 Å and c = 6.38 Å (Fig. 5b). In this structure, two A and A′ layers having a point symmetry relation, exhibit alternating stacking of amine-type and imine-type N atoms. The simulated PXRD pattern reproduces the observed diffraction pattern (Fig. 5a).
a Powder X-ray diffraction patterns of CuHAB-1 (red), CuHAB-10 (green), and CuHAB-100 (blue) and simulated diffraction patterns of P31c space-group structure (brown) and Cm space-group structure (black). Chemical structures of b CuHAB-1 (P31c space group, a = 13.2 Å, c = 6.38 Å) and c CuHAB-10 and CuHAB-100 (Cm space group, a = 21.17 Å, b = 13.48 Å, c = 3.52 Å, β = 99.20°)
CuHAB-10 and CuHAB-100 exhibit a shift of peak positions from 14.3° in CuHAB-1 to 14.6° and an appearance of additional peaks at 4.4°, 8.9°, 12.2°, and 13.1° compared with CuHAB-1, suggesting a different periodic structure of CuHAB when synthesized with a high Na2CO3 concentration. This diffraction pattern is not reproduced well by the reported structures of HAB-based coordination nanosheets synthesized by the O2-oxidation method, which adopts P6/mmm, Cmcm, and C2221 space groups [15, 18]. The PXRD-simulated patterns using the Cm space group with cell parameters of a = 21.17 Å, b = 13.48 Å, c = 3.52 Å, and β = 99.20° can reproduce the experimental results of CuHAB-10 and CuHAB-100 (Figs. 5a and c). In this structure, each CuHAB layer is stacked with a shift in the a-axis direction (Fig. 5c). Possible factors that cause CuHAB films to adopt different stacking structures are the interlayer interaction derived from hydrogen bonding and the interlayer electric repulsion around the Cu complex. In CuHAB-1, the hydrogen atom in an amino group can form a hydrogen bond between the nitrogen atoms in the layers above and beneath, generating an interlayer interaction to stabilize the AA’ stacking. On the other hand, most of nitrogen atoms in CuHAB-10 and CuHAB-100 assume the imino structure, and the interlayer interaction derived from hydrogen bonds is weakened. In addition, XPS measurements revealed that CuHAB-10 and CuHAB-100 have higher ratios of Cu(I) species than CuHAB-1. The increase of Cu(I), which has a higher electron density in d orbitals than Cu(II) enhances the interlayer electric repulsion between copper centers in different layers. Hence, CuHAB-10 and CuHAB-100 form slipped stacking structures to decrease layer-to-layer electric repulsion by avoiding the overlap of Cu complexes in the neighboring layers.
3.6 Humidity Responsive Conductivity
Electrical properties of CuHAB-X films were investigated by the two-probe method, using Au interdigitated electrodes under ambient conditions (Fig. 6). CuHAB-10 has the highest conductivity of (1.1 ± 0.9) × 10−3 S cm−1, which is two-orders of magnitude greater than those of CuHAB-1 ((2.3 ± 1.5) × 10−5 S cm−1) and CuHAB-100 ((6.2 ± 4.1) × 10−5 S cm−1). The observed conductivity of CuHAB-10 is comparable to previously reported values for pelletized CuHAB [16]. A possible reason for the low conductivity of CuHAB-1 and CuDI-100 is the existence of amino structure in CuHAB-1 and the number of grain boundaries in CuHAB-100. As described above, the IR and XP spectra suggest that CuHAB-1 has nitrogen atoms taking the amino structure. The copper complex unit in CuHAB-1, composed of ligated amino groups cannot form a quasi-aromatic system like the bis(diimino)copper complex, and the π-conjugation system is divided to reduce the electric conductivity of CuHAB, resulting in lower conductivity of CuHAB-1 than CuHAB-10. Our previous study on bis(aminothiolato)nickel (NiAT) and bis(iminothiolato)nickel (NiIT) nanosheets indicates that NiIT is more favorable than NiAT in basic conditions and the conductivity of NiAT is much lower than that of NiIT [23]. On the other hand, CuHAB-100 is composed of small crystalline domains, as evidenced by TEM, so that there are a large number of domain boundaries, reducing the electronic conductivity. As an additional point, CuHAB-1 exhibits humidity-responsive conductivity, which increases under 75% RH, while it decreases under 10% RH (Fig. 6b). The presence of amino groups and the porous structure of CuHAB-1 can contribute to this humidity response by production of hydrogen bonding points and pathways for acquisition/loss of water molecules, respectively. CuHAB-10 and CuHAB-100 did not exhibit a significant enhancement of conductivity when humidity increased from ambient to 75% RH (Fig. S9). The report of a humidity response of amorphous MHAB films by Ding and co-workers claims that defect sites in the films produce multiple hydroxyl groups, acting as hydrogen bonding sites with H2O molecules and enhancing the response performance [14]. The present result explains that the introduction of amino groups as hydrogen bonding sites allows crystalline CuHAB to exhibit humidity-response conductivity change.
4 Conclusion
This study utilized liquid–liquid interfacial synthesis to synthesize CuHAB with the quantitative addition of an oxidant and Na2CO3 as a base. X-ray diffraction studies of CuHAB films obtained using various benzoquinone derivatives revealed that an oxidizing agent with low oxidation potential is appropriate for crystalline CuHAB formation. The morphology and chemical and crystal structures of CuHAB-X films formed at the H2O/dichloromethane interface with tBu2BQ and Na2CO3 were investigated using AFM, TEM, IR, XPS, and PXRD. According to these characterizations, CuHAB-10 and CuHAB-100 have a slipped stacking structure expressed as a Cm space group, which is a new stacking mode for HAB-based coordination frameworks. Meanwhile, CuHAB-1 assumes the AA’ stacking structure produced by interlayer interaction derived from hydrogen bonds between vertically stacked amino and imino groups. CuHAB-10 exhibits the highest conductivity at 1.1 × 10−3 S cm−1 under ambient conditions, while CuHAB-1 shows a moisture response with a conductivity increase at high humidity (75% RH). These results indicate that the liquid–liquid interfacial technique with an appropriate oxidizing agent and quantitative addition of base is a powerful way to modulate the chemical and stacking structure of HAB-based coordination nanosheets and contributes to their various chemical functions and phenomena.
References
A. Schneemann, R. Dong, F. Schwotzer, H. Zhong, I. Senkovska, X. Fen, S. Kaskel, 2D framework materials for energy applications. Chem. Sci. 12, 1600–1619 (2021). https://doi.org/10.1039/D0SC05889K
M. Wang, R. Dong, X. Feng, Two-dimensional conjugated metal–organic frameworks (2D c-MOFs): chemistry and function for MOFtronics. Chem. Soc. Rev. 50, 2764–2793 (2021). https://doi.org/10.1039/D0CS01160F
J. Liu, X. Song, T. Zhang, S. Liu, H. Wen, L. Chen, 2D conductive metal-organic frameworks: an emerging platform for electrochemical energy storage. Angew. Chem. Int. Ed. 60, 5612–5624 (2021). https://doi.org/10.1002/anie.202006102
L.S. Xie, G. Skorupskii, M. Dincă, Electrically conductive metal-organic frameworks. Chem. Rev. 120, 8536–8580 (2020). https://doi.org/10.1021/acs.chemrev.9b00766
M. Ko, L. Mendecki, K.A. Mirica, Conductive two-dimensional metal–organic frameworks as multifunctional materials. Chem. Commun. 54, 7873–7891 (2018). https://doi.org/10.1039/C8CC02871K
K. Wada, K. Sakaushi, S. Sasaki, H. Nishihara, Multielectron-transfer-based rechargeable energy storage of two-dimensional coordination frameworks with non-innocent ligands. Angew. Chem. Int. Ed. 57, 8886–8890 (2018). https://doi.org/10.1002/anie.201802521
K. Wada, H. Maeda, T. Tsuji, K. Sakaushi, S. Sasaki, H. Nishihara, Tailoring the electrochemical properties of two-dimensional Bis(diimino)metal coordination frameworks by introducing Co/Ni heterometallic structures. Inorg. Chem. 59, 10604–10610 (2020). https://doi.org/10.1021/acs.inorgchem.0c01055
J. Park, M. Lee, D. Feng, Z. Huang, A.C. Hinckley, A. Yakovenko, X. Zou, Y. Cui, Z. Bao, Stabilization of hexaaminobenzene in a 2D conductive metal-organic framework for high power sodium storage. J. Am. Chem. Soc. 140, 10315–10323 (2018). https://doi.org/10.1021/jacs.8b06020
M. Amores, K. Wada, K. Sakaushi, H. Nishihara, Reversible energy storage in layered copper-based coordination polymers: unveiling the influence of the ligand’s functional group on their electrochemical properties. J. Phys. Chem. C 124, 9215–9224 (2020). https://doi.org/10.1021/acs.jpcc.0c01486
D. Feng, T. Lei, M.R. Lukatskaya, J. Park, Z. Huang, M. Lee, L. Shaw, S. Chen, A.A. Yakovenko, A. Kulkarni, J. Xiao, K. Fredrickson, J.B. Tok, X. Zou, Y. Cui, Z. Bao, Robust and conductive two-dimensional metal−organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 3, 30–36 (2018). https://doi.org/10.1038/s41560-017-0044-5
S.C. Wechsler, F.Z. Amir, Superior electrochemical performance of pristine nickel hexaaminobenzene MOF supercapacitors fabricated by electrophoretic deposition. Chemsuschem 13, 1491–1495 (2019). https://doi.org/10.1002/cssc.201902691
B. Cui, C. Wang, S. Huang, L. He, S. Zhang, Z. Zhang, M. Du, Efficient multifunctional electrocatalyst based on 2D semiconductive bimetallic metal-organic framework toward non-Pt methanol oxidation and overall water splitting. J. Colloid Interface Sci. 578, 10–23 (2020). https://doi.org/10.1016/j.jcis.2020.05.098
C. Li, L. Shi, L. Zhang, P. Chen, J. Zhu, X. Wang, Y. Fu, Ultrathin two-dimensional π–d conjugated coordination polymer Co3(hexaaminobenzene)2 nanosheets for highly efficient oxygen evolution. J. Mater. Chem. A 8, 369–379 (2020). https://doi.org/10.1039/C9TA10644H
C. Liu, Y. Gu, C. Liu, S. Liu, X. Li, J. Ma, M. Ding, Missing-linker 2D conductive metal organic frameworks for rapid gas detection. ACS Sens. 6, 429–438 (2021). https://doi.org/10.1021/acssensors.0c01933
J.-H. Dou, L. Sun, Y. Ge, W. Li, C.H. Hendon, J. Li, S. Gul, J. Yano, E.A. Stach, M. Dincă, Signature of metallic behavior in the metal-organic frameworks M3(hexaiminobenzene)2 (M = Ni, Cu). J. Am. Chem. Soc. 139, 13608–13611 (2017). https://doi.org/10.1021/jacs.7b07234
A.C. Hinckley, J. Park, J. Gomes, E. Carlson, Z. Bao, Air-stability and carrier type in conductive M3(Hexaaminobenzene)2, (M = Co, Ni, Cu). J. Am. Chem. Soc. 142, 11123–11130 (2020). https://doi.org/10.1021/jacs.0c03500
S.S. Shinde, C.H. Lee, J.-Y. Jung, N.K. Wagh, S.-H. Kim, D.-H. Kim, C. Lin, S.U. Lee, J.-H. Lee, Unveiling dual-linkage 3D hexaiminobenzene metal–organic frameworks towards long-lasting advanced reversible Zn–air batteries. Energy Environ. Sci. 12, 727–738 (2019). https://doi.org/10.1039/C8EE02679C
E.J.H. Phua, K.-H. Wu, K. Wada, T. Kusamoto, H. Maeda, J. Cao, R. Sakamoto, H. Masunaga, S. Sasaki, J.-W. Mei, W. Jiang, F. Liu, H. Nishihara, Oxidation-promoted Interfacial synthesis of redox-active bis(diimino)nickel nanosheet. Chem. Lett. 47, 126–129 (2018). https://doi.org/10.1246/cl.170928
Z.-G. Tao, X. Zhao, X.-K. Jiang, Z.-T. Li, A hexaazatriphenylene-based organogel that responds to silver(I) with high selectivity under aqueous condition. Tetrahedron Lett. 53, 1840–1842 (2012). https://doi.org/10.1016/j.tetlet.2012.01.137
J. Mahmood, D. Kim, I.-Y. Jeon, M.S. Lah, J.-B. Baek, Scalable synthesis of pure and stable hexaaminobenzene trihydrochloride. Synlett 24, 246–248 (2013). https://doi.org/10.1055/s-0032-1317696
K. Kato, Y. Tanaka, M. Yamauchi, K. Ohara, T. Hatsui, A statistical approach to correct X-ray response non-uniformity in microstrip detectors for high-accuracy and high-resolution total-scattering measurements. J. Synchrotron Radiat. 26, 762–773 (2019). https://doi.org/10.1107/S1600577519002145
M.T. Huynh, C.W. Anson, A.C. Cavell, S.S. Stahl, S. Hammes-Schiffer, Quinone 1 e– and 2 e–/2 H+ reduction potentials: identification and analysis of deviations from systematic scaling relationships. J. Am. Chem. Soc. 138, 15903–15910 (2016). https://doi.org/10.1021/jacs.6b05797
X. Sun, K.-H. Wu, R. Sakamoto, T. Kusamoto, H. Maeda, X. Ni, W. Jiang, F. Liu, S. Sasaki, H. Masunaga, H. Nishihara, Bis(aminothiolato)nickel nanosheet as a redox switch for conductivity and an electrocatalyst for the hydrogen evolution reaction. Chem. Sci. 8, 8078–8085 (2017). https://doi.org/10.1039/C7SC02688A
Acknowledgements
This work was financially supported by JST-CREST JPMJCR15F2, JSPS KAKENHI (Grant Number: JP19H05460, JP20K15242, JP22K14569, and JP22K05055), and TOYOTA MOTOR CORPORATION. The synchrotron radiation experiments were performed at BL44B2 (PXRD, Proposal No. 20200054) and BL05XU (GIXS) in SPring-8 (Hyogo, Japan). XPS measurements were supported by the University of Tokyo Advanced Characterization Nanotechnology Platform in the Nanotechnology Platform Project sponsored by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (JPMXP09-A-20-UT-0365). TEM observations were supported by Prof. Y. Idemoto (Department of Pure and Applied Chemistry, Tokyo University of Science) and Dr. T. Ichihashi (Research Equipment Centre, Tokyo University of Science).
Funding
Open access funding provided by Tokyo University of Science.
Author information
Authors and Affiliations
Contributions
HM conducted experiments and prepared the main manuscript and figures. KT conducted XPS, PXRD, and GIXS measurements. NF conducted powder XED and GIXS experiments. HM, SS, and KK set up the measurement systems for PXRD and GIXS in SPring-8. HM, JU, NH, and HN periodically discussed the research progress. HN supervised the research project. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maeda, H., Takada, K., Fukui, N. et al. Manipulation of Chemical and Crystal Structures of Multilayered Copper-Hexaaminobenzene Coordination Nanosheets to Modulate Conductivity with Precise Control of Liquid–Liquid Interfacial Synthesis. J Inorg Organomet Polym (2023). https://doi.org/10.1007/s10904-023-02920-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-023-02920-5