Abstract
The biosynthesis of metal nanoparticles using plant extracts is an eco-friendly and inexpensive solution that has strong potential and applications in science and industry. This study aims to synthesize Cu, Ag, and Au monometallic and trimetallic nanoparticles (NPs) using the extracted polysaccharides (PS) of Vossia cuspidata (Roxb.) Griff. leaves. Besides, the anti-cancer, anti-inflammatory, and wound healing potentials of the synthesized NPs were tested. The synthesized NPs were characterized using standard technological methods. We succeeded in green synthesizing CuO, Ag, Au, monometallic, and CuO-Ag-Au trimetallic NPs. The synthesized NPs had weak cytotoxicity at low concentrations (6.5 µg/ml), but the viability of cancer cells was reduced by increasing the concentration, suggesting that the synthesized NPs have potent anti-cancer properties against the cells. The synthesized NPs had 19.44–45.9 μg/ml cytotoxic activity (IC50) against the MCF-7 cell line, 16.50–51.92 μg/ml against A549, and 115.90–165.9 μg/ml for normal lung cells (WI-38). TMNPs were the most effective cytotoxic agents against all the tested cell lines, followed by AuNPs on MCF-7 and CuONPs on A549. The cotton fabric-treated TMNPs and CuONPs exhibited anti-inflammatory properties greater than fabric-treated AgNPs and AuNPs and showed the highest odema inhibition (84.61% and 79.28%, respectively). In the wound healing assay, CuONPs and TMNPs caused the highest percentages of inhibition (87.82% and 61.98%, respectively) for the wound compared to AgNPs and AuNPs. TMNPs and CuONPs were more efficient in restoring the tissue integrity of wounds than AgNPs and AuNPs. Accordingly, we recommend using TMNPs and CuONPs in the wound healing dressings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal and metal oxide NPs are divided into mono-, bi-, tri-, and quadrometallic types according to the number of metals. Bi- and tri-metallic NPs exhibit more improved catalytic capabilities and advantageous features as compared to monometallic NPs, Trimetallic nanoparticles can be created utilizing a variety of techniques and reducing agents, Colloidal TMNPs are an important class of multifunctional nanoscale materials that support diverse applications as biological imaging, cancer therapy, magnetic, sensors, solar energy conversion, sensors, optics, sensors and catalytic characteristics [1,2,3,4,5,6,7,8,9]. The incorporation of a metal or many metals into the NPs structure improves the overall total electronic charge shift, deduction of the lattice distance, and changes in the electron structure [10]. Monometallic NPs, such as those made of Ag, Au, Zn, and Cu, are composed of a single type of metal with distinct chemical and physical characteristics. The antibacterial properties of monometallic and monometallic oxide NPs are well recognized. Ag, Au, and CuO NPs stand out among them due to their potent antibacterial properties against a variety of pathogens [11]. Bimetallic and bimetallic oxide nanoparticles, such as Ag/Au, Ag/Cu, Cu/Zn, Au/CuS, and CuO/ZnO, frequently have distinctive antibacterial properties. Trimetallic NPs display higher catalytic selectivity, activity, and efficiency in a variety of applications. Due to their multifunctional actions, trimetallic NPs have novel physicochemical properties [12]. They consequently received greater scientific attention recently and gained popularity in technological and medical applications such as catalytic processes and wound healing [13, 14].
Green synthesis is highly desired even if there are numerous physical and chemical ways to make various kinds of metals and metal oxides. It provides cheap and eco-friendly flexible solutions [15]. During the synthesis of metal NPs, plant extracts are known to act as reducing and stabilizing agents [16]. This may be attributed to secondary metabolites in plant extracts that reduce metal ions and enhance the formation of NPs [17, 18]. Medicinal plants were a promising target for the production of NPs due to their beneficial medicinal compounds. Many metals were synthesized as NPs using plant extracts, particularly Cu, Ag, and Au which are known for their antibacterial activity [19,20,21]. Copper is one of the important metals in our bodies and it regulates many mechanisms for cytokine and growth factors, and hence it is important in wound healing and as an antimicrobial agent [22]. Numerous research suggested that gold nanoparticles (AuNPs) have antibacterial and anticancer properties [19, 20, 23]. Silver (Ag) has a principal role as an antibacterial agent. When AgNPs are mixed with other metals, such as CuONPs or AuNPs, they exhibit more effective antibacterial characteristics than pure Ag or CuONPs [24, 25]. At low concentrations, Ag exhibited anti-inflammatory and antimicrobial activities and is used in wound healing applications [26]. The manufacture of wound dressings with antimicrobial and anti-inflammatory agents is a valuable measure to control microbial colonization and infection in wounds [27].
Although plant extracts are frequently utilized to produce monometallic NPs, there hasn’t been much global study on the environmentally friendly production of trimetallic NPs. Vossia cuspidata (Hippo grass in English) is a perennial emergent macrophyte of the Poaceae family [28]. Although the plant is rarely studied in its distribution areas, it is a promising forage and medicinal plant that has many important constituents such as proteins, carbohydrates, glucosides, alkaloids, terpenoids, and flavonoids, in addition to a valuable essential oil [29,30,31,32,33]. The biosynthesis of CuO/ Ag/ Au monometallic and CuO-Ag-Au trimetallic nanoparticles using the extracted PS of V. cuspidata leaves was not yet reported. The objectives of this study were to (1) synthesize CuO/ Ag/ Au monometallic and trimetallic NPs using the PS of the aqueous leaf extract of V. cuspidata and their characterization, (2) evaluate the anti-cancer, anti-inflammatory, and wound healing potentials of the synthesized NPs.
Fourier transform infrared (FTIR), X-ray diffraction (XRD), Ultraviolet–Visible (UV–vis) absorption spectroscopy, and transmission electron microscopy (TEM) were used to characterize the produced NPs.
2 Material and Methods
2.1 Chemicals and Reagents
Silver nitrate (AgNO3), copper sulphate heptahydrate (CuSO4.7H2O), chloroauric acid (HAuCl4·3H2O), and carrageenan were purchased from Sigma-Aldrich. Sodium hydroxide from El Nasr Pharmaceutical Chemicals Company, Cairo, Egypt. These chemicals were of high analytical grade. El-Mahalla Company for weaving and spinning generously donated cotton fabrics. Silver sulphadiazine ointment (S.S. ointment) 1% was purchased from Kahira Pharm. Ind. Co. supplied (A.R.E.). The main methodological steps are shown in Fig. 1.
2.2 Plant Sampling, Extraction, and Hydrolysis of Polysaccharides
The leaves of V. cuspidata were collected from its natural habitats in Cairo, Egypt. To remove dust particles and any impurities, leaves of V. cuspidata were washed with tap water followed by distilled water. Shade-dried leaves were powdered using an electric grinder and then stored in sealed vials in the refrigerator until further extraction.
50 gm of shade powdered leaves were placed in 200 ml of distilled water and heated at 100 °C using a hot-plate until complete extraction. Using a rotavapor apparatus, the extract was concentrated to a volume of around 50 mL, after which 250 ml of absolute ethanol was added drop by drop with stirring until full precipitation of polysaccharide was achieved. After cleaning with ethanol (100%), the residue (polysaccharides) was weighed and kept at 4–10 °C.
To identify the type of monosaccharides that constitute the polysaccharides residue, 0.1 gm of polysaccharides residue was treated with 10 ml of 1 N HCl for 5 h in a boiling water bath. After hydrolysis, the solution was treated with Ba (OH)2, centrifuged, and the precipitate was washed twice with water before being evaporated until the volume was reduced to 2 ml. Ethyl acetate was partitioned three times and stored in vials until it was injected into the high-performance liquid chromatography (HPLC) apparatus (Agilent VWD Series Germany). Analysis of monosaccharides using HPLC was according to the manufacturer’s guidelines.
2.3 Biosynthesis of Monometallic Nanoparticles
The synthesis of monometallic CuONPs, AgNPs, and AuNPs was carried out as follows:
To synthesize CuONPs, 0.1 gm of PS was added to 100 ml of distilled H2O, and the temperature was adjusted to 70 °C. Then 1 ml of Cu salt solution (0.1 M) was added to the previous solution, then the mixture was stirred for 60 min. until the color of the solution changed from yellow color to a brown color. The resulting colloidal solution was oven-dried at 70 °C to obtain CuONPs.To synthesize AgNPs, 0.1 gm of the PS was dissolved in 100 ml distilled H2O and the temperature was raised to 70 °C. Then, the 1 ml of Ag salt solution (0.1 M) was added to the PS solution, and the mixture was stirred continuously for 60 min. with a magnetic stirrer. The final color of the colloidal solution was reddish brown.
To synthesize AuNPs 0.1 gm of the PS was dissolved in 100 ml distilled H2O and the temperature was raised to 70 °C. Then, the 1 ml of Au salt solution (0.1 M) was added to the PS solution, keeping the temperature at 70 °C for 60 min with continuous stirring until developing of violet colloidal solution [34, 35].
2.4 Biosynthesis of Trimetallic Nanoparticles (TMNPs)
The trimetallic nanoparticles Cu-Ag-Au were synthesized by dissolving 0.3 g of PS in 100 ml of distilled H2O. Then equal volumes of Cu, Ag, and Au metal precursor (1:1:1) solutions were added to the PS solution under continuous stirring at 70 ◦C until the formation of a colloidal solution.
The conversion of metal ions to NPs was initially confirmed using a UV–visible spectrophotometer. To collect the solid materials for further characterization and confirmation of the trimetallic and monometallic nanoparticle formation, the colloidal NP solutions were dried separately at 100 °C. The dry solid materials were stored in a dry place for further characterization and biological applications.
2.5 Characterization of the Biosynthesized Nanoparticles
The bio-reduction of copper oxide, silver, and gold ions was recorded periodically using a T80 UV–Vis double beam spectrometer (PG Instruments Ltd., UK). The biosynthesized dried Ag, Au, and Cu monometallic and trimetallic nanoparticles were characterized using XRD spectroscopy (XPERT–PRO–P analytical–Netherland), FTIR spectroscopy (FT-IR spectrometer 6100 JASCO), range of 400–4000 cm−1), with a resolution of 4 cm−1, Scanning Electron Microscopy (SEM) (type JXA-840A, Japan), Transmission Electron Microscopy (TEM) (JEOL JEM-2100, Japan) were used for characterization and measurements of the synthesized nanoparticles. Each sample was prepared as follows: 2 ml of the synthesized nanoparticles were diluted to 10 ml, sonicated using a sonicator water bath, and then 3 drops of it were placed on a Cu grid with ultrathin Cu on holey C-film, and then allowed to be dried in a vacuum. The instrument was operated with an acceleration voltage of 200 kV. All the instruments and devices were used according to the manufacturer’s guidelines and settings.
2.6 In Vitro Biological Activities
2.6.1 Anti-Cancer (MTT) Assay
Breast cancer cells (MCF-7), lung cancer cells (A549), and diploid normal cells (WI-38) were cultured in RPMI-1640 and Dulbico medium supplemented with 10% foetal bovine serum (56 °C), l- glutamine (3 mM), streptomycin (100 mg/ml), penicillin (100 IU/ml), and 25 mM 4-aminolevulinic acid (2-Hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) Cells were grown at 37 °C in a humidified atmosphere with 95% air and 5% CO2.
The stock solutions of Nanoparticle (10 mg/ml) were diluted in DMSO to reach the needed working concentrations and subsequently dissolved in a suitable medium. In 96-well flat bottom microplates, all cancer cells and normal cells (5000 cells per well) were seeded for 24 h before being treated with five different doses of tenfold diluted nanoparticle for 48 h later. The final concentrations delivered to target cells were 100, 50, 25, 12.5, and 6.5 μg/ml, and 0 for control wells, which merely gave the cells the nutritional media. For another 48 hours, cells were treated with various doses of nanoparticles, with Doxorubicin serving as a positive control.
Cell survival was measured 72 h after the nanoparticles were added using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test). In phosphate buffered saline, each well received 50 μl of MTT solution (5 mg/ml) (PBS). Afterward, the samples were cultured for an additional four hours. The insoluble product formazan (purple color) produced by living cells converting MTT dye and the yellow color produced by dead cells were then added to 100 μl of Dimethyl sulfoxide. The number of viable cells in each well was proportional to the intensity of light absorbance, which was read in an enzyme-linked immunosorbent assay (ELISA) Biotek plate reader (ELX-800) at 570 nm, to determine cell survival (%). The A of the blank was always expected to be subtracted from the A of the equivalent sample with target cells. In comparison to a vehicle-treated control, the half-maximum inhibitory concentration (μg/ml) was defined as the half-maximum inhibitory concentration (IC50). All tests were performed three times in total.
2.6.2 Anti-Inflammatory Activity
In the presence of a 1% binder (printofix R binder MTBEG liquid), cotton textiles were treated with 2% monometallic and trimetallic nanoparticles. In the finishing bath, the cotton fabric was padded to a 100% wet pick-up, then dried at 80 °C for 3 min before curing at 140 °C for 3 min. The anti-inflammatory properties of cotton fabric treated with different NPs and their ten washes was investigated. As an anti-inflammatory drug assay, carrageenan caused oedema in the rat’s hind paw. The study protocol was conducted according to the ethical considerations of the International Committee on Laboratory Animals (ICLA). A total of 36 albino male rats were split into six groups (G6) of six individuals each. Animals were anesthetized with anesthetic ether and had their backs shaved with electric clippers using an open mask approach. The six groups were divided as follows: G1: represents the blank. G2, G3, and G4 represent cotton fabrics treated with CuONPs, AgNPs, and AuNPs and their 10 washes, respectively. Then, they were firmly tied to the back skin. G5 represents the cotton fabrics that were treated with TMNPs and their 10 washes and firmly tied to the back skin. Lastly, G6 represents the blank tissue under which a drug standard (S.S. Ointment) was applied and firmly tied.
After an hour of treatment, all animals received a subplantar injection in their right hind paw of 0.1 ml of carrageenan-saline solution (1%, w/v) in their left hind paw. The scarification of the rats occurred four hours after the application of the films. The two hind paws were removed and weighted individually after two, three, and four hours of scarification of the rats.
The Oedema percentage and oedema inhibition percentage were calculated according to the following equations [36, 37],
where: Mc = the mean % oedema in the control group, Mt = the mean % oedema in the drug-treated group.
2.6.3 Wound Healing
Pad–dry–cure was used to incorporate CuONPs, AgNPs, AuNPs, and TMNPs into cotton fabrics. Fabrics (20 cm × 20 cm) were immersed in a solution bath and compressed to 100% wet pick-up using a laboratory pad at continuous pressure to produce NPs-cellulosic fabrics. For thermal fixation of nanoparticles on fabric surfaces, the samples were dried at 80 °C for 5 min and cured at 120 °C for 3 min.
The following is a brief description of the washing procedure for CuONPs, AgNPs, AuNPs, and TMNPs coated fabrics: The fabric was washed in a solution containing 2 g/l Na2CO3 and 1 ml commercial detergent. The sample was then mixed and left at 50 ± 5 °C for 20 min. Finally, the fabrics were gently squeezed and rinsed under running water. To obtain ten washings, this method was performed 10 times.
Male Sprague–Dawley rats weighing 130–150 g were procured from the National Research Center’s animal house in Cairo, Egypt. The animals were kept in the same sanitary circumstances and fed a conventional laboratory diet. All animals were handled according to the recommendations of the institutional ethical committee (National Research Center, Cairo, Egypt).
After wound creation, the experimental animals were divided into six groups (G) as follows: G1 in which a blank, G2 in which cotton fabrics were treated with CuONPs and their 10 washes, respectively, G3 had cotton fabrics treated with AgNPs and their 10 washes, G4 had cotton fabrics treated with AuNPs and their 10 washes, G5 had cotton fabrics treated with TMNPs and their 10 washes, G5 had drug standard S.S. Ointment that was applied to the blank tissue on film and then all six group were firmly tied to the skin of the back.
Wound contraction was studied using excision wounds. Full-thickness skin was removed in the dorsal interscapular area in all incisions. The rats were anesthetized, and their backs were shaved with electric clippers using an open mask technique. Cutting pieces of skin from the shaved area, excision incisions of about 2 cm2 were created. The wounds were covered with the appropriate bandage. Dressings were changed daily. As dressings were changed, wounds were examined, photographed, and measured. The progressive change in healing was measured in the wound area (mm2). Wound contraction was expressed as inhibition % in original wound size.
The epithelization period was described as the number of days needed for the scab to completely fall off and leave no trace of the raw wound.
2.7 Statistical Analysis
From six animals in each group, the results were presented as a mean value with its standard deviation (mean ± S.E.). The ANOVA test was used to determine statistical significance, followed by a post hoc Duncan multiple range test. Statistical significance was defined as a probability value (p) < 0.05.
3 Results and Discussion
3.1 HPLC Analysis of V. cuspidata Polysaccharides
Eight sugars were identified by HPLC examination of V. Cuspidata hydrolysates, accounting for 92.39% of the total. Galactose and glucose were the highest sugar fractions with 33.6% and 22.3%, respectively. In contrast, sorbitol and xylose had the lowest percentage (2.4% and 3.2%, respectively) (Table 1). HPLC findings revealed the presence of several sugars containing ketone or aldehyde groups, which were responsible for the reduction of metal ions and stabilization of NPs. Qualitative analysis carried out by Raphael et al. [30] indicated the presence of many phytochemicals including carbohydrates, glycosides, alkaloids, terpenoids, and flavonoids.
3.2 Characterization of the Produced NPs
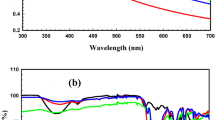
The use of UV–vis spectroscopy to confirm the generation and stabilization of synthesized NPs is very important. CuONPs, AgNPs, AuNPs, and TMNPs have UV absorption peaks of 250 nm for CuONPs, 410 nm for AgNPs, and 545 nm for AuNPs, respectively, where TMNPs have two absorption peaks of 405 and 255 nm (Fig. 2a).
An FT-IR spectroscopy device is a useful tool for detecting the functional groups that can be utilized to reduce metal ions to metal nanoparticles and improve the stability of biosynthesized nanoparticles. When these biomolecules are discovered, new ways of nanoparticle synthesis can be developed [28, 29]. The surface chemistry of nanoparticles also has an impact on their properties and uses. In all spectra, the bands at 3500–3200 cm−1 were assigned to O–H stretching vibration, the bands at 2850–3000 were assigned to C-H stretching, 1700–1850 cm−1 bands were assigned to the carbonyl group, 1600–1650 cm−1 alkenyl aliphatic C=C stretch, 1400–1600 cm−1-alkenyl aromatic C=C stretch, 1350–1401.73 cm−1(C–H bending), vibration at 1078.26–1300 cm−1 –C–O group [30,31,32]. The biomolecules in the PS extract converted metal ions into metal NPs, resulting in nanoparticle stability and a decrease in bands [33] as presented in Fig. 2b.
Figures 3a–i represented the unwashed and washed cotton fabric samples. Nanosized CuONPs, AgNPs, AuNPs, and TMNPs were found on the cotton fabric surface in both washed and unwashed samples, NPs were greater in the unwashed sample compared to the washed sample. Both times, the NPs were evenly distributed. The nanoparticles were well dispersed in both cases, although some visible particles were still aggregated across the fabric surface. The produced NPs were also evenly, smoothly, and uniformly distributed across the fabric surface. They were evenly dispersed and densely populated on cotton fabric samples. It is clear from SEM pictures that NPs were strongly attracted to the textiles because of their chemical interaction with each other. According to the findings, the NPs finished cotton fabric had a wash fastness of 10 washes.
The XRD for CuONPs, AgNPs, AuNPs, and TMNPs that were synthesized with PS of V. Cuspidata are shown in Fig. 4. The crystalline NPs were identified in the XRD spectrum not only by their peak positions but also by their relative intensities. The crystallinity of the produced CuONPs was revealed by XRD, with peaks at 2θ values 32.98°, 35.23°, 38.67°, 49.25°, 58.32°, and 61.99° corresponding to lattice planes 110, 002, 111, 202, 020, 202, and 311, respectively [34, 35]. The XRD pattern corresponds to the monoclinic phase of CuONPs and JCPDS card no. (89-2531). XRD measurements were used also to confirm the crystallinity of biosynthesized AuNPs and AgNPs. Five diffraction peaks were visible in the XRD patterns of AuNPs that correspond to 111, 200, 220, 311, and 222 of metal gold at 2θ = 37.52°, 45.25°, 65.7°, 77.67°, and 82.88°, respectively [36, 37]. These powerful peaks were confirmed by JCPDS Card No. 021095. On the other hand, the AgNPs, displayed a similar diffraction profile, with peaks at 2θ of 38.12°, 45.37°, 65.99°, 77.07°, and 82.48°, respectively, which may be attributed to the face-centered cubic silver crystals’ 111, 200, 220, 311, and 222 crystallographic planes [38, 39]. Diffraction peaks in the TMNPs spectrum are found at 2θ values of 34.98°, 35.23°, 48.62°, and 61.99° corresponding to lattice planes 110, 002, 111, and 113 of copper oxide NPs, respectively [34, 40], and 38.67°, 45.01°, 68.14°, 77.34°, and 82.31°, corresponding to lattice planes 111, 200, 220, 311, and 222 of silver and gold NPs [37, 38, 40].
The TEM technique was used to visualize the morphology, particle size, and number of formed NPs. TEM is also a method of producing photos by illuminating the sample with electronic radiation (under vacuum) and detecting the electrons that are transmitted through the sample [41, 42] In the present study, the prepared NPs (Fig. 5a–d) had a spherical shape with particle sizes ranging from 5 to 12 nm for CuONPs, 3 to 8 nm for AgNPs, 10 to 20 nm for AuNPs, and R9 to 19 nm for TMNPs. CuONPs and AgNPs had a minor agglomeration, while AuNPs and TMNPs had a good distribution as reported by other investigators [43].
3.3 Anti-Cancer Activity
The anti-cancer effect of monometallic and trimetallic nanoparticles was evaluated using a dose-dependent inhibitory activity against two cancer cell lines. Many drugs are toxic to the body and have serious side effects. As a result, alternative green-generated chemotherapeutic drugs containing CuONPs, AgNPs, AuNPs, and TMNPs were developed [44]. In this study, the cytotoxicity of CuONPs, AgNPs, AuNPs, and TMNPs was investigated using the cell viability (%) of MCF-7, A549, and WI-38 cells (Table 2). Synthesized CuONPs, AgNPs, AuNPs, and TMNPs demonstrated weak cytotoxicity at an initial concentration (6.5 µg/ml). When the concentration of the synthesized NPs (CuONPs, AgNPs, AuNPs, and TMNPs) was increased, cancer cell viability was reduced, suggesting that the synthesized NPs have potent anti-cancer properties against the cells. CuONPs, AgNPs, AuNPs, TMNPs, and doxorubicin had cytotoxic activity (IC50) against MCF-7 cell line of 45.95, 56.83, 35.40, 19.44, and 16.51 μg/ml, respectively. In addition, the cytotoxic agents had IC50 values of 18.24, 51.92, 48.82, 16.50, and 10.61 μg/ml, respectively, against A549. The IC50 values for normal lung cells (WI-38) were 122.3, 115.9, 118.24, 165.9, and 220.6 μg/ml, respectively. Based on these findings, we can conclude that TMNPs are the most effective cytotoxic agents against the tested cell lines, followed by AuNPs on MCF-7 and CuONPs on A549. At all tested doses, the results showed that NPs had a high cell vitality in normal lung cells. They were non-toxic and TMNPs were the safest, as demonstrated in Table 2. The anti-cancer properties of Au, Ag, and CuO NPS as monometallic or bimetallic NPs were reported by many investigators such as Zhou et al. and Hossen et al. [16, 45]. TMNPs have high catalytic activity compared to their bimetallic or monometallic nanoparticles [46]. The potential for synergistic effects is one of the most significant advantages of combination therapy. In combination therapy, the total therapeutic benefit of nanoparticles was found to be greater than the sum of the advantages of nanoparticles alone [47,48,49].
3.4 Anti-Inflammatory Activity
The TMNPs and CuONPs treated fabric exhibited anti-inflammatory properties more than AgNPs and AuNPs treated fabric (Table 3). TMNPs showed the highest odema inhibition (84.61%), followed by CuONPs (79.28%). The unwashed fabric exhibited odema inhibition more than the washed fabric. The results showed that washing only destroyed a small percentage of the NPs and the treated fabric had a strong resistance to the washing process, which is consistent with the SEM results. Hashem et al., Abou Zeid et al., and Faisal et al. [26, 27, 50] reported similar findings. The paw diameter (mm) was significantly different between all the tested groups at zero time, 1, 2, 3 and 4 h (at p < 0.05, Table 3). Inflammation may appear after trauma, infection, post-ischemic injury, poisoning, or autoimmune disease harms a tissue. The interactions between soluble chemicals and cells are intricate. The process typically leads to healing and infection recovery [26]. Carrageenan-induced inflammation is nonimmune, acute, and causes the classic symptoms of inflammation, such as edoema, hyperalgesia, and erythema, to appear right away after a subcutaneous injection. These pro-inflammatory substances include bradykinin, histamine, complement, tachykinins, reactive oxygen and nitrogen species, and histamine. Such substances may be produced naturally at the location of the injury or by invading cells [50].
3.5 Wound Healing Activity
Wound healing is a dynamic process in which the skin responds to it starting with an accumulation of platelets, coagulation, and inflammation, and ending with re‐epithelization [51]. Inflammation happens during the wound healing process to help in the elimination of the contaminating microorganisms. Wound healing or contraction is a parameter that indicates the reduction rate of the unhealed area during the treatment course. The efficacy of the medication was evaluated according to the reduction of the wound (% inhibition). SEM revealed that a certain amount of CuONPs, AgNPs, AuNPs, and TMNPs was released from the fabric after the washing process and there is no significant change in the percentage of wound contraction in the case of washed/unwashed cotton. The supplementary figures (Figs. S1 and S2) showed some photographs of the wound area during the treatment process at zero, 4 days, and 10 days for different washed and unwashed samples. Table 4 showed that the unwashed samples have an inhibition percentage better than that of the washed samples. CuONPs and TMNPs caused the highest inhibition percentages for the wound compared to AgNPs and AuNPs. The percentage of inhibition of TMNPs was 87.82% followed by its 10 washes (85.05%) and CuONPs (61.98%). It seems that TMNPs and CuONPs were efficient as anti-inflammatory and anti-microbial agents and helped in restoring wound tissue integrity more than Ag and Au NPs. CuONPs play a critical role in living cells whereas it regulates cytokines and growth factor mechanisms and contributes to all processes of wound healing [14, 52]. Alizadeh et al. [53] reported that a 1 mM concentration of 80 nm CuONPs was very effective for wound healing treatments without negative side effects. Compared to monometallic and bimetallic nanoparticles, trimetallic NPs offer unique structural and chemical features due to their synergistic or multifunctional effects [54]. Accordingly, cotton fabric treatment with TMNPs was preferable to achieve excellent wound contraction, as reported by other investigators such as Sundaramoorthi et al. and Krishnan et al. [55, 56].
4 Conclusions
In this study, we were able to prepare CuO, Ag, Au monometallic, and trimetallic CuO-Ag-Au NPs using the extracted polysaccharides of leaves of V. cuspidata leaves. The synthesized monometallic and trimetallic NPs were well characterized by using the standard technological methods. The synthesized NPs had weak cytotoxicity at low concentrations (6.5 µg/ml), but the viability of cancer cells was reduced by increasing the concentration, suggesting that the synthesized NPs have potent anti-cancer properties against the cells. Based on our findings, we can conclude that TMNPs are the most effective cytotoxic agents against all tested cell lines, followed by AuNPs on MCF-7 and CuONPs on A549. The cellulose fabric treated with TMNPs and CuONPs exhibited anti-inflammatory properties more than cellulose fabric treated with AgNPs and AuNPs. TMNPs showed the highest inhibition of odema (84.61%), followed by CuONPs (79.28%). The unwashed fabric exhibited odema inhibition more than the washed fabric. The findings revealed that washing only destroyed a small percentage of the NPs, and the treated fabric was highly resistant to washing. When the synthesized NPs were used in the wound healing assay, the unwashed samples had an inhibition percentage better than that of the washed ones. CuONPs and TMNPs caused the highest inhibition percentages for wounds compared to AgNPs and AuNPs. It seems that TMNPs and CuONPs were efficient as anti-inflammatory and anti-microbial agents and helped in restoring wound tissue integrity more than Ag and Au NPs. Accordingly, we recommend using TMNPs and CuONPs in wound dressings. Further exploration of the applications of TMNPs in medicine and other science and industry fields is recommended.
Data Availability
All data generated or analyzed during this study are included in this published article and no supplementary files are attached to the mns.
References
G. Sharma, A. Kumar, S. Sharma, M. Naushad, R. Prakash Dwivedi, Z.A. ALOthman, G.T. Mola, J. King Saud Univ. Sci. 31, 257–269 (2019)
B. Karthikeyan, B. Loganathan, Mater. Lett. 85, 53–56 (2012)
N. Yadav, A.K. Jaiswal, K. Dey, V.B. Yadav, G. Nath, A. Srivastava, R. Yadav, Mater. Chem. Phys. 218, 10–17 (2018)
B. Loganathan, B. Karthikeyan, Colloids Surf. A 436, 944–952 (2013)
B. Loganathan, V.L. Chandraboss, M. Murugavelu et al., J Sol-Gel Sci Technol 74, 1–14 (2015)
V.K. Chaturvedi, N. Yadav, N.K. Rai et al., Environ Sci Pollut Res 28, 13761–13775 (2021)
N. Yadav, P. Chaudhary, K.K. Dey et al., J Mater Sci: Mater Electron 31, 17843–17854 (2020)
V. Chaturvedi, S. Rai, N. Tabassum, N. Yadav, V. Singh, R. Bohara, M. Singh, Biochem. Biophys. Rep. 24, 1–7 (2020)
N. Yadav, R.R. Yadav, K.K. Dey, J. Alloy. Compd. 896, 163073 (2022)
F.L. Deepak, A. Mayoral, R. Arenal, Adv. Transm. Electron. Microsc. Appl. Nanomater. 1–272 (2015).
D.S. Karaman, S. Manner, A. Fallarero, J.M. Rosenholm, Antibact. Agents (2017).
S. Ali, A.S. Sharma, W. Ahmad, M. Zareef, M. Hassan, A. Viswadevarayalu, T. Jiao, H. Li, Q. Chen, Crit. Rev. Anal. Chem. (2020). https://doi.org/10.1080/10408347.2020.1743964
G. Sharma, V.K. Gupta, S. Agarwal, S. Bhogal, M. Naushad, A. Kumar, F.J. Stadler, J. Mol. Liq. 260, 342–350 (2018)
R. Dobrucka, Int. J. Environ. Anal. Chem. 101(14), 2046–2057 (2021). https://doi.org/10.1080/03067319.2019.1691543
M.R. Kamli, M.A. Malik, S.A. Lone, J.S. Sabir, E.H. Mattar, A. Ahmad, Pharmaceutics 13(11), 1957 (2021)
P.B. Ashishie, C.A. Anyama, A.A. Ayi, C.O. Oseghale, E.T. Adesuji, A.H. Labulo, Int. J. Phys. Sci. 13(3), 24–32 (2018). https://doi.org/10.18483/ijSci.1637
X. Weng, M. Guo, F. Luo, Z. Chen, Chem. Eng. J. 308, 904–911 (2017). https://doi.org/10.1016/j.cej.2016.09.134
A. Velidandi, N.P.P. Pabbathi, S. Dahariya, R.R. Baadhe, Nano-Struct. Nano-Objects 26, 100687 (2021)
Z. Sun, W. Zheng, G. Zhu, J. Lian, J. Wang, P. Hui, S. He, W. Chen, X. Jiang, A.C.S. Appl, Mater. Interfaces 11(49), 45381–45389 (2019)
M. Tanaka, M. Hayashi, L. Roach, Y. Kiriki, T. Kadonosono, T. Nomoto, N. Nishiyama, J. Choi, K. Critchley, S.D. Evans, M. Okochi, Acta Biomater. 131, 519–531 (2021)
Q. Song, Y. Liu, P. Zhang, W. Feng, S. Shi, N. Zhou, X. Chu, J. Shen, A.C.S. Appl, Nano Mater. 5, 8621–8630 (2022)
A.P. Kornblatt, V.G. Nicoletti, A. Travaglia, J. Inorg. Biochem. 161, 1–8 (2016)
I.H. El-Sayed, X. Huang, M.A. El-Sayed, Cancer Lett 239(1), 129–135 (2006)
Q. Zhou, L. Zhang, H. Wu, Nanotechnol. Rev. 6, 473–496 (2017)
S. Cheeseman, A.J. Christofferson, R. Kariuki, D. Cozzolino, T. Daeneke, R.J. Crawford, V.K. Truong, J. Chapman, A. Elbourne, Adv. Sci. 7, 1902913 (2020)
F. Paladini, M. Pollini, Materials 12, 2540 (2019). https://doi.org/10.3390/ma12162540
I. Negut, V. Grumezescu, A.M. Grumezescu, Molecules 23, 2392 (2018)
L. Boulos, Al Hadara Publishing, Cairo, Egypt, pp. 617 (2005).
M. Runde, D. Kubmarawa, H.M. Maina, Res. J. Chem 5(10), 7–12 (2015)
E. Raphael, S. Momoh, D. Kayode, A. Gideon, E.T. Friday, Int. Curr. Res. Biosci. Plant Biol. 3(8), 53–57 (2016)
W.F. Mahmoud, E.A. Farahat, G.M. Fahmy, H.F. Farrag, H.E.A. Awad, Taeckholmia 41, 1–17 (2021)
W.F. Mahmoud, E.A. Farahat, G.M. Fahmy, H.F. Farrag, H.E. Awad, Aquat. Bot. 172, 103399 (2021)
E. Farahat, G. Fahmy, H. Farrag, W. Mahmoud, H. Awad, Egypt. J. Bot. (2021). https://doi.org/10.21608/ejbo.2021.98322.1796
A.H. Abou Zeid, M.S. Hifnawy, R.S. Mohammed, J Med Aromat Plants 3, 42–49 (2009)
H.M. El-Rafie, S.M. Abd El-Aziz, M.K. Zahran, Der Pharm Lett 8(19), 156–164 (2016)
S.M. AbdEl-Aziz, A.A. Sleem, M.I.A. Abdel Maksoud. Cellulose (2022).
S. Faisal, H. Jan, I. Abdullah Alam, M. Rizwan, Z. Hussain, K. Sultana, Z. Ali, M.N. Uddin, ACS Omega 7(5), 4071–4082 (2022). https://doi.org/10.1021/acsomega.1c05410
F. Faghihzadeh, N.M. Anaya, L.A. Schifman et al., Nanotechnol. Environ. Eng. 1, 1 (2016). https://doi.org/10.1007/s41204-016-0001-8
P. Khandel, S. Shahi, L. Kanwar, R. Yadaw, D. Kumar Soni, Int. J. Nano Dimens. 9, 273–285 (2019)
M.J. Firdhouse, P. Lalitha, Asian J. Pharm. Clin. Res. 6, 92–94 (2012)
A. Masri, A. Anwar, D. Ahmed, R.B. Siddiqui, M. Raza Shah, N.A. Khan, J. Antibiot. 7(4), 100 (2018). https://doi.org/10.3390/antibiotics7040100
M. Aslam, F. Fozia, A. Gul, I. Ahmad, R. Ullah, A. Bari, R.A. Mothana, H. Hussain, Molecules 26(20), 6144 (2021)
T. Kokila, P. Ramesh, D. Geetha, Appl. Nanosci. 5, 911–920 (2015)
D. Zhu, L. Wang, W. Yu, H. Xie, Sci. Rep. 8, 5282 (2018). https://doi.org/10.1038/s41598-018-23174-z
S. Mureed, S. Naz, A. Haider et al., Nanoscale Res Lett 16, 91 (2021). https://doi.org/10.1186/s11671-021-03547-6
S. Krishnamurthy, A. Esterle, N.C. Sharma et al., Nanoscale Res Lett 9, 627 (2014). https://doi.org/10.1186/1556-276X-9-627
A. Biswas, Orient J Chem 37(5), 1187–1191 (2021)
P. Prakash, P. Gnanaprakasam, R. Emmanuel, S. Arokiyaraj, M. Saravanan, Colloids Surf. B: Biointerfaces 108, 255–259 (2013)
V. Maragoni, M.A.D. Ayodhya, A. Madhusudhan, V. Guttena, Int. J. Green Nanotechnol. Biomed. 4, 199–206 (2018). https://doi.org/10.1080/19430892.2012.705999
Z. Chen, D. Mochizuki, M.M. Maitani, Y. Wada, Nanotechnol. 24, 265602 (2013). https://doi.org/10.1088/0957-4484/24/26/265602
B.J. Inkson, Wood head Publishing, Book, pp. 17–43 (2016). https://doi.org/10.1016/B978-0-08-100040-3.00002-X.
R. Esmail, A. Afshar, M. Morteza et al., BMC Microbiol. 22, 97 (2022). https://doi.org/10.1186/s12866-022-02490-5
L. Xiong, T. He, Chem. Mater. 18(9), 2211–2218 (2006). https://doi.org/10.1021/cm052320t
M. Greenwell, P.K. Rahman, J. Pharm. Sci. Res. 6(10), 4103–4112 (2015). https://doi.org/10.13040/IJPSR.0975-8232.6(10).4103-12
S. Hossen, M.K. Hossain, M.K. Basher, M. Mia, M.T. Rahman, M.J. Uddin, J. Adv. Res. 15, 1–18 (2018)
K.H. Huynh, X.H. Pham, J. Kim, S.H. Lee, H. Chang, W.Y. Rho, B.H. Jun, Int. J. Mol. Sci. 21, 5174 (2020). https://doi.org/10.3390/ijms21145174
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SMAEA: conceptualization, study design, methodology, data analysis, data curation, writing the original draft, reviewing, and editing. EAF: conceptualization, collection of plant materials, writing the original draft, reviewing, editing, and supervising the whole work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest and also declares that they have no financial interests.
Ethical Approval
The current experimental design was approved by the research ethical committee of the Zoology Department, Helwan University (approval no. HU-IACUC/Ch/ASM0122-2), following the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, 8th Edition (NIH Publication no. 85–23, revised 1985).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Aziz, S.M., Farahat, E.A. The Activity of Vossia cuspidata Polysaccharides-Derived Monometallic CuO, Ag, Au, and Trimetallic CuO-Ag-Au Nanoparticles Against Cancer, Inflammation, and Wound Healing. J Inorg Organomet Polym 33, 853–865 (2023). https://doi.org/10.1007/s10904-023-02542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02542-x