Abstract
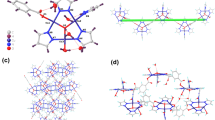
The reaction of potassium tetracyanocupprate(I) with triethyltin bromide in presence of phenanthroline (Phen) and quinoxaline (Qox) in acetonitrile via ultrasonic radiation yielded two new nano-supramolecular coordination polymers (SCP) of the type: [Cu2(CN)4(Et3Sn)2(Phen)2] SCP1 and [Cu2(CN)3(Et3Sn)(Qox)] SCP2. The obtained polymers were characterized using elemental and thermal analyses, as well as FT-IR, UV–vis, fluorescence, 1H NMR, and MS spectroscopes. Spectral and analytical features led to the conclusion that both Phen and Qox behave as bidentate ligands and the proposed formulae of SCP1 and SCP2 have the bimetallic nature, in which the geometry around Cu(I) atoms in SCP1 is tetrahedral and planar trigonal. While in SCP2, the Cu(I) has a trigonal plane geometry. Thermal analysis studies indicated that both SCP1 and SCP2 are thermally stable up to 140 °C. Additional confirmation for the structures of SCP1 and SCP2 was obtained from density functional theory (DFT) and molecular mechanics (MM+) calculations. Transmission electron microscopy (TEM) images of SCP1 and SCP2 show regular spherical-like nano-sized particles in the range of 31.24–62.13 nm and 5.16–23.90 nm, respectively. The inhibitory anti-oxidant activities of both SCP1 and SCP2 were investigated using erythrocyte hemolysis and ABTS methods and their cytotoxicity activities towards different tumor cells were also studied by MTT assay. The results showed that both the two polymers have high inhibitory anti-oxidant activities and SCP2 exhibits a significant decrease in surviving fraction of these cancer cell lines compared to SCP1.










Similar content being viewed by others
References
D. Braga, F. Grepioni, Chem. Commun. (1996). https://doi.org/10.1039/CC9960000571
A.S. Abd-El-Aziz, P.O. Shipman, B.N. Boden, W.S. McNeil, Prog. Polym. Sci. 35, 714 (2010) and references therein
S. Silver, FEMS Microbiol. Rev. 27, 341 (2003)
M.J.M. Vaerewijck, G. Huys, J.C. Palomino, J. Swings, F. Portaels, FEMS Microbiol. Rev. 29, 911 (2005)
E.S. Raper, Coord. Chem. Rev. 153, 199 (1996)
S. Zartilas, S.K. Hadjikakou, N. Hadjiliadis, N. Kourkoumelis, L. Kyros, M. Kubicki, M. Baril, I.S. Butler, S. Karkabounas, J. Balzarini, Inorg. Chim. Acta 362, 1003 (2009)
M. Cavicchioli, A.C. Massabni, T.A. Heinrich, C.M. Costa-Neto, E.P. Abrảo, B.A.L. Fonseca, E.E. Castellano, P.P. Corbi, W.R. Lustri, C.Q.F. Leite, J. Inorg. Biochem. 104, 533 (2010)
E.E. Tredget, H.A. Shankowsky, A. Groenveld, R. Burrell, J. Burn Care Rehabil. 19, 531 (1998)
S.E.H. Etaiw, S.N. Abdou, A.A. Faheim, J. Coord. Chem. 68, 491 (2015)
K.R. Vinothkumar, R. Henderson, Q. Rev. Biophys. 43, 65 (2010)
K.R. Siebenlist, F. Taketa, Toxicol. Appl. Pharmacol. 58, 67 (1981)
A.A. Ali, R.K. Upreti, A.M. Kidway, Toxicol. Lett. 38, 13 (1987)
A.A. Ali, R.K. Upreti, A.M. Kidway, Bull. Environ. Contam. Toxicol. 44, 29 (1990)
J.H. Bang, K.S. Suslick, Adv. Mater. 22, 1039 (2010)
B.M. Elliot, W.N. Aldridge, J.W. Bridges, Biochem. J. 177, 461 (1979)
S.E.H. Etaiw, M.M. El-bendary, Spectrochim. Acta A 110, 304 (2013)
M. Gielen, E.R.T. Tiekink, Metallotherapeutic Drug and Metal-Based Diagnostic Agents: 50Sn Tin Compounds and Their Therapeutic Potential (Wiley, New York, 2005), pp. 421–439
N. Ogwuru, L.E. Khoo, G. Eng, Appl. Organomet. Chem. 12, 409 (1998)
G. Sava, G. Jaouen, E.A. Hillard, A. Bergamo, Dalton Trans. 41, 8226 (2012)
J.M. Hearn, I. Romero-Canelón, B. Qamar, Z. Liu, I. Hands-Portman, P.J. Sadler, ACS Chem. Biol. 8, 1335 (2013)
A. Hottin, D.W. Wright, A. Steenackers, P. Delannoy, F. Dubar, C. Biot, G.J. Davies, J.-B. Behr, Chem. Eur. J. 19, 9526 (2013)
M.M. Meier, C. Rajendran, C. Malisi, N.G. Fox, C. Xu, S. Schlee, D.P. Barondeau, B. Höcker, R. Sterner, F.M. Raushel, J. Am. Chem. Soc. 135, 11670 (2013)
M.A. Jakupec, M. Galanski, V.B. Arion, C.G. Hartinger, B.K. Keppler, Dalton Trans. (2008). https://doi.org/10.1039/B712656P
K. Strohfeldt, M. Tacke, Chem. Soc. Rev. 37, 1174 (2008)
C.G. Hartinger, P.J. Dyson, Chem. Soc. Rev. 38, 391 (2009)
L. Pellerito, L. Nagy, Coord. Chem. Rev. 224, 111 (2002)
V. Dokorou, A. Primikiri, D. Kovala-Demertzi, J. Inorg. Biochem. 105, 195 (2011)
M. Gielen, E.R.T. Tiekink, Tin compounds and their therapeutic potential, in Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine. ed. by M. Gielen, E.R.T. Tiekink (Wiley, West Sussex, 2005), pp. 421–439
A.M.A. Ibrahim, E. Siebel, R.D. Fischer, Inorg. Chem. 37, 3521 (1998)
E. Siebel, A.M.A. Ibrahim, R.D. Fischer, Inorg. Chem. 38, 2530 (1999)
H. Hanika-Heidl, S.E.H. Etaiw, MSh. Ibrahim, R.D. Fischer, A.S. Badr El-din, J. Organomet. Chem. 684, 329 (2003)
S.E.H. Etaiw, S.N. Abdou, J. Inorg. Organomet. Polym. 20, 622 (2010)
M. Zurro, S. Asmus, S. Beckendorf, C.M. Lichtenfeld, O.G. Mancheno, J. Am. Chem. Soc. 136, 13999 (2014)
B.A. Maynard, R.E. Sykora, J.T. Maguec, A.E.V. Gorden, Chem. Commun. 46, 4944 (2010)
M.L. Toma, R. Lescouëzec, F. Lloret, M. Julve, J. Vaissermann, M. Verdaguer, Chem. Commun. 1850 (2003)
E. Lissi, B. Modak, R. Torres, J. Escobar, A. Urza, Free Radic. Res. 30, 471 (1999)
A.B.A. El-Gazzar, A.M.S. Youssef, M.M. Youssef, A.A. Abu-Hashem, F.A. Badria, Eur. J. Med. Chem. 44, 609 (2009)
R. Aeschlach, J. Loliger, C.B. Scott, A. Murcia, J. Butler, B. Halliwel, I.O. Aruoma, Food Chem. Toxicol. 32, 31 (1994)
Y. Morimoto, K. Tanaka, Y. Iwakiri, S. Tokuhiro, S. Fukushima, Y. Takeuchi, Biol. Pharm. Bull. 18, 1417 (1995)
T. Mosmann, J. Immunol. Methods 65, 55 (1983)
F. Denizot, R. Lang, J. Immunol. Methods 22, 271 (1986)
R.A. Penneman, L.H. Jones, J. Chem. Phys. 24, 293 (1956)
S.E.H. Etaiw, S.N. Abdou, Appl Organomet. Chem. 32, 4053 (2018)
H.H. Jalfe, M. Orechin, Theory and Applications of Ultraviolet Spectroscopy, 5th edn. (Wiley, New York, 1970)
N. Armaroli, L.D. Cola, V. Balzani, J.-P. Sauvage, C. Dietrich-Buchecker, J.-M. Kern, J. Chem. Soc. Faraday Trans. 88, 553 (1992)
B.N. Bandyopadhyay, A. Harriman, J. Chem. Soc. Faraday Trans. 1 73, 663 (1977)
G.M. Badger, I.S. Walker, J. Chem. Soc. (1956). https://doi.org/10.1039/JR9560000122
K. Yamamote, T. Takemura, H. Baba, Bull. Chem. Soc. Jpn 51, 729 (1978)
M.J. Lim, C.A. Murray, T.A. Tronic, K.E. deKrafft, A.N. Ley, J.C. deButts, R.D. Pike, H. Lu, H.H. Patterson, Inorg. Chem. 47, 6947 (2008)
C.A. Bayse, T.P. Brewster, R.D. Pike, Inorg. Chem. 48, 174 (2009)
S.E.H. Etaiw, S.A. Amer, M.M. El-Bendary, J. Inorg. Organomet. Polym. 21, 662 (2011)
B. Delley, J. Chem. Phys. 92, 508 (1990)
B. Delley, Int. J. Quantum Chem. 69, 433 (1998)
A. Kessi, B. Delley, Int. J. Quantum Chem. 68, 135 (1998)
X. Wu ana, A.K. Ray, Phys. Rev. B 65, 85403 (2002)
Materials, Studio v 5.0 Copyright 2009 (Accelrys Software, Inc., 2009)
W.J. Hehre, L. Radom, P.V.R. Schleyer, J.A. Pople, Ab Initio Molecular Orbital Theory (Wiley, New York, 1986)
B. Hammer, L.B. Hansen, J.K. Nørskov, Phys. Rev. B 59, 7413 (1999)
A. Matveev, M. Staufer, M. Mayer, N. Rösch, Int. J. Quantum Chem. 75, 863 (1999)
S.E.H. Etaiw, S.N. Abdou, A.S. Badr El-din, J Inorg. Organomet. Polym. 25, 1478 (2015)
C. Pellerito, L. Nagy, L. Pellerito, A. Azorcsik, J. Organomet. Chem. 691, 1733 (2006)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdou, S.N. Ultrasonic Assisted Nano-structures of Novel Organotin Supramolecular Coordination Polymers as Potent Antitumor Agents. J Inorg Organomet Polym 31, 3962–3975 (2021). https://doi.org/10.1007/s10904-021-02055-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02055-5