Abstract
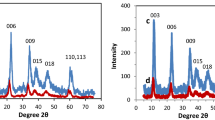
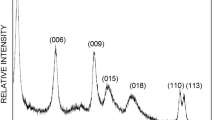
The Zn2–Al layered double hydroxides (LDHs) have been used to remove the 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) herbicide, which is used as a model for pesticides from the carboxylate family. The adsorption isotherms are governed by Langmuir model (L type). The characterization by various techniques (XRD, FTIR, SEM) indicates that the 2,4,5-T retention is governed by adsorption on the sites of the LDH surface when the molar ratio 2,4,5-T/Cl ≤ 0.1. For greater ratio values, the herbicide undergoes, in addition, intercalation in the LDH interlayer domain leading to an increase of the interlayer distance from 0.778 to 1.855 nm. The retention capacity reaches 718 mg/g with a removal rate of 96% for an optimal 2,4,5-T/Cl molar ratio of 0.5. This capacity is influenced by the solution pH, the charge density of the LDH sheets, the adsorbent/adsorbate molar ratio and the nature of the interlayer anion of the LDH precursor. The partial or total release of the herbicide was observed to depend on the composition of the desorbing solution. This suggests the possibility of LDH recycling and confirms thus its effectiveness in eliminating this type of pollutant from water. In addition, a well-structured [Zn2–Al-2,4,5-T] hybrid material was obtained after herbicide retention and its characterization by chemical analyses and SEM as well as a proposed structural model are presented.












Similar content being viewed by others
References
S.M. Bradberry, A.T. Proudfoot, A. Vale, Poisoning due to chlorophenoxy herbicides. Toxicol. Rev. 23(2), 65–73 (2004)
R.S. Chhokar, S. Singh, R.K. Sharma, Herbicides for control of isoproturon-resistant littleseed canarygrass (Phalaris minor) in wheat. Crop Prot. 27, 719–726 (2008)
D. Wang, F.N.D. Mukome, D. Yan, H. Wang, K.M. Scow, S.J. Parikh, Phenylurea herbicides sorption to biochars and agricultural soil. J. Environ. Sci. Health B 50, 544–551 (2015)
S. Giacomazzi, N. Cochet, Environmental impact of diuron transformation: a review. Chemosphere 56, 1021–1032 (2004)
A. Silkina, A. Brazes, F. Vouve, V. Le Tilly, P. Douzenel, J.L. Mouget, N. Bourgougnon, Antifouling activity of macroalgal extracts on Fragilaria pinnata (Bacillariphyceae): a comparison with diuron. Aquat. Toxicol. 94, 245–254 (2009)
S.J. Kalkhoff, D.W. Kolpin, E.M. Thurman, I. Ferrer, D. Barcelo, Degradation of chloroacetanilide herbicides: the prevalence of sulfonic and oxanilic acid metabolites in Iowa groundwaters and surface waters. Environ. Sci. Technol. 32, 1738–1740 (1998)
A. Pandiarajan, R. Kamaraj, S. Vasudevan, OPAC (orange peel activated carbon) derived from waste orange peel for the adsorption of chlorophenoxyacetic acid herbicides from water: Adsorption isotherm, kinetic modelling and thermodynamic studies. Bioresour. Technol. 261, 329–341 (2018)
H. Kim Hue, L.V. Anh, D.B. Trong, Study of the adsorption of 2,4-dichlorophenoxyacetic acid from the aqueous solution onto activated carbon. Vietnam J. Chem. 56(2), 208–213 (2018)
I.K. Konstantinou, T.A. Albanis, Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways. Appl. Catal B 42, 319–335 (2003)
X. Zhu, C. Yuan, Y. Bao, J. Yang, Y. Wu, Photocatalytic degradation of pesticide pyridaben on TiO2 particles. Int. Nano Lett. 229, 95–105 (2005)
M.I. Badawy, M.Y. Ghaly, T.A. Gad-Allah, Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 194, 166–175 (2006)
M.V. Phanikrishna Sharma, V. Durga Kumari, V.M. Subrahmanyam, TiO2 supported over SBA-15: an efficient photocatalyst for the pesticide degradation using solar light. Chemosphere 73, 1562–1569 (2008)
N.T. Kim Phuong, M.W. Beak, B.T. Huy, Y.I. Lee, Adsorption and photodegradation kinetics of herbicide 2,4,5-trichlorophenoxyacetic acid with MgFeTi layered double hydroxides. Chemosphere 146, 51–59 (2019)
S. Khalaf, J.H. Shoqeir, L. Scrano, R. Karaman, S.A. Bufo, T.A. Kurniawan, Removal of herbicides from water using heterogeneous photocatalysis case study: MCPA sodium monohydrate. J. Water Resour. Prot. 11, 1024–1035 (2019)
S.F.A. Shattar, N.A. Zakaria, K.Y. Foo, Acid modified natural clay as a judicious solution for the successive treatment of ametryn. Desalin. Water Treat. 103, 270–279 (2018)
J.S. Calisto, I.S. Pacheco, L.L. Freitas, L.K. Santana, W.S. Fagundes, F.A. Amaral, S.C. Canobre, Adsorption kinetic and thermodynamic studies of the 2,4–dichlorophenoxyacetate (2,4-D) by the [Co–Al–Cl] layered double hydroxide. Heliyon 5, e02553 (2019)
A. Iglesias, R. López, D. Gondar, J. Antelo, S. Fiol, F. Arce, Adsorption of MCPA on goethite and humic acid-coated goethite. Chemosphere 78, 1403–1408 (2010)
M. Eshete, J. Bowleg, S.G. Perales, M. Okunrobo, D. Watkins, H. Spencer, Adsorption of propazine, simazine and bisphenol A on the surface of nanoparticles of iron oxide nanoparticles of carbon and metallic oxides. J. Environ. Prot. 9, 13–24 (2018)
J. Shah, M.R. Jan, I. Rahman, Dispersive solid phase microextraction of fenoxaprop-p-ethyl herbicide from water and food samples using magnetic graphene composite. J. Inorg. Organomet. Polym. 30, 1716–1725 (2020)
A. Derylo-Marczewska, M. Blachnio, A.W. Marczewski, A. Swiatkowski, B. Tarasiuk, Adsorption of selected herbicides from aqueous solutions on activated carbon. J. Therm. Anal Calorim. 101, 785–794 (2010)
W.T. Tsai, H.R. Chen, Adsorption kinetics of herbicide paraquat in aqueous solution onto a low-cost adsorbent, swine-manure-derived biochar. Int. J. Environ. Sci. Technol. 10, 1349–1356 (2013)
S.R. Shanmugam, S. Adhikari, H. Nam, V. Patil, Adsorption and desorption behavior of herbicide using bio-based materials. Trans. ASABE 62(6), 1435–1445 (2019)
R. Kamaraj, S. Vasudevan, Facile one-pot electrosynthesis of Al(OH)3—kinetics and equilibrium modeling for adsorption of 2,4,5-trichlorophenoxyacetic acid from aqueous solution. New J. Chem. 40, 2249–2258 (2016)
A.L. Gimsing, O.K. Borggaard, Effect of phosphate on the adsorption of glyphosate on soils, clay minerals and oxides. Int. J. Environ. Anal. Chem. 82, 545–552 (2002)
E. Morillo, T. Undabeytia, A. Cabrera, J. Villaverde, C. Maqueda, Effect of soil type on adsorption−desorption, mobility, and activity of the herbicide norflurazon. J. Agric. Food Chem. 52, 884–890 (2004)
I.A. Ololade, N.A. Oladoja, F.F. Oloye, F. Alomaja, D.D. Akerele, J. Iwaye, P. Aikpokpodion, Sorption of glyphosate on soil components: the roles of metal oxides and organic materials. Soil Sediment Contam. 23(5), 571–585 (2014)
E.A.O. Pereira, V.F. Melo, G. Abate, J.C. Masini, Adsorption of glyphosate on Brazilian subtropical soils rich in iron and aluminum oxides. J. Environ. Sci. Health B 54, 906–914 (2019)
M.H.B. Hayes, U. Mingelgrin, Interactions between small organic chemicals and soil colloidal constituents, in Interactions at the soil colloid-soil solution interface. ed. by G.H. Boyd et al. (Kluwer Academic Publishers, Dordrecht, 1991), pp. 323–407
A. Ghafoor, N.J. Jarvis, T. Thierfelder, J. Stenstrom, Measurements and modelling of pesticide persistence in soil at the catchment scale. Sci. Total Environ. 409, 1900–1908 (2011)
K.E. Hall, C. Ray, S.J. Ki, K.A. Spokas, W.C. Koskinen, Pesticide sorption and leaching potential on three Hawaiian soils. J. Environ. Manage. 159, 227–234 (2015)
J.P.K. Gill, N. Sethi, A. Mohan, Analyses of the glyphosate herbicide in water, soil and food using derivatising agents. Environ. Chem. Lett. 15, 85–100 (2017)
M. Hagner, J. Mikola, I. Saloniemi, K. Saikkonen, M. Helander, Effects of a glyphosate-based herbicide on soil animal trophic groups and associated ecosystem functioning in a northern agricultural field. Sci. Rep. 9, 8540 (2019)
F. Lai, Y. Huang, Y. Miao, T. Liu, Controllable preparation of multi-dimensional hybrid materials of nickel-cobalt layered double hydroxide nanorods/nanosheets on electrospun carbon nanofibers for high-performance supercapacitors. Electrochim. Acta. 74, 456–463 (2015)
A. Bialas, M. Mazur, P. Natkanski, B. Dudeka, M. Kozak, A. Wacha, P. Kustrowski, Hydrotalcite-derived cobalt–aluminum mixed oxide catalysts for toluene combustion. Appl. Surf. Sci. 362, 297–303 (2016)
A.J. Khoei, N.J.G. Joogh, P. Darvishi, K. Rezaei, Application of physical and biological methods to remove heavy metal, arsenic and pesticides, malathion and diazinon from water. Turk. J. Fish. Aquat. Sci. 19, 21–28 (2019)
H. Pourfarzad, M. Shabani-Nooshabadi, M.R. Ganjali, H. Kashani, Synthesis of Ni–Co–Fe layered double hydroxide and Fe2O3/Graphene nanocomposites as actively materials for high electrochemical performance supercapacitors. Electrochim. Acta. 317, 83–92 (2019)
N. Ye, N. Cimetiere, V. Heim, N. Fauchon, C. Feliers, D. Wolbert, Upscaling fixed bed adsorption behaviors towards emerging micropollutants in treated natural waters with aging activated carbon: model development and validation. Water Res. 148, 30–40 (2019)
V. Chivu, D. Gilea, N. Cioatera, G. Carja, M. Mureseanu, Heterostructures of Ce–Ti/layered double hydroxides and the derived MMOs for photoenergy applications. Appl. Surf. Sci. 513, 145853 (2020)
L. Cocheci, L. Lupa, N.S. Ţolea, C. Muntean, P. Negrea, Sequential use of ionic liquid functionalized Zn–Al layered double hydroxide as adsorbent and photocatalyst. Sep. Purif. Technol. 250, 117104 (2020)
A.V. Gorokhovsky, A.R. Tsiganov, T.V. Nikityuk, J.I. Escalante-Garcia, I.N. Burmistrov, V.G. Goffman, Synthesis and properties of nanocomposites in the system of potassium polytitanate—layered double hydroxide. J. Mater. Res. Technol. 9, 3924–3934 (2020)
H. Nabipour, Design and evaluation of non-steroidal anti-inflammatory drug intercalated into layered zinc hydroxide as a drug delivery system. J. Inorg. Organomet. Polym. 29, 1807–1817 (2019)
Y.H. Kotp, Enhancement of industrial effluents quality by using nanocomposite Mg/Al LDH ultrafiltration membranes. J. Inorg. Organomet. Polym. 30, 5244–5260 (2020)
J. Yu, K. Ruengkajorn, D.G. Crivoi, C. Chen, J.C. Buffet, D. O’Hare, High gas barrier coating using non-toxic nanosheet dispersions for flexible food packaging film. Nat. Commun. 10, 2398 (2019)
R. Zhang, Y. Ai, Z. Lu, Application of multifunctional layered double hydroxides for removing environmental pollutants: recent experimental and theoretical progress. J. Environ. Chem. Eng. 8, 103908 (2020)
J. Das, B.S. Patra, N. Baliarsingh, K.M. Parida, Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 32, 252–260 (2006)
H. Zazou, N. Oturan, H. Zhang, M. Hamdani, M.A. Oturan, Comparative study of electrochemical oxidation of herbicide 2,4,5-T: kinetics, parametric optimization and mineralization pathway. Sustain. Environ. Res. 27, 15–23 (2017)
B. Bukowska, 2,4,5-T and 2,4,5-TCP induce oxidative damage in human erythrocytes: the role of glutathione. Cell Biol. Int. 28, 557–563 (2004)
S. Miyata, The syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties-I: the systems Mg2+-Al3+-NO3-, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4-, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 23, 369–375 (1975)
G. Fan, W. Sun, H. Wang, F. Li, Visible-light-induced heterostructured Zn–Al–In mixed metal oxide nanocomposite photocatalysts derived from a single precursor. Chem. Eng. J. 174, 467–474 (2011)
G.H. Eshaq, A.M. Rabie, A.A. Bakr, A.H. Mady, A.E. ElMetwally, Cr(VI) adsorption from aqueous solutions onto Mg-Zn-Al LDH and its corresponding oxide. Desalin. Water Treat. 57, 20377–20387 (2016)
E. Elkhattabi, M. Lakraimi, M. Berraho, A. Legrouri, R. Hammal, L. El Gaini, Acid Green 1 removal from wastewater by layered double hydroxides. Appl. Water Sci. 8, 45 (2018)
N. Iyi, T. Matsumoto, Y. Kanek, K. Kitamura, Deintercalation of carbonate ions from a hydrotalcite-like compound: enhanced decarbonation using acid-salt mixed solution. Chem. Mater. 16(15), 2926–2932 (2004)
Z. Yan, B. Zhu, J. Yu, Z. Xu, Effect of calcination on adsorption performance of Mg–Al layered double hydroxide prepared by a water-in-oil microemulsion method. RSC Adv. 6, 50128–50137 (2016)
M.A. Ahmad, N.A.A. Puad, O.S. Bello, Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour. Ind. 6, 18–35 (2014)
K. Abdellaoui, I. Pavlovic, M. Bouhent, A. Benhamou, C. Barriga, A comparative study of the amaranth azo dye adsorption/desorption from aqueous solutions by layered double hydroxides. Appl. Clay Sci. 143, 142–150 (2017)
G. Crini, P.M. Badot, Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog. Polym. Sci. 33, 399–447 (2008)
C.H. Giles, T.H. MacEwan, S.N. Nakhwa, D. Smith, Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 14, 3973–3993 (1960)
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
A. Angelova, C. Ringard-Lefebvre, A. Baszkin, Drug–cyclodextrin association constants determined by surface tension and surface pressure measurements: I. Host–guest complexation of water soluble drugs by cyclodextrins: polymyxin B–β cyclodextrin system. J. Colloid Interface Sci. 212, 275–279 (1999)
R.V. Prikhodko, M.V. Sychev, I.M. Astrelin, K. Erdmann, A. Mangel, R.A. Santen, Synthesis and structural transformations of hydrotalcite like materials Mg-Al and Zn-Al. Russ. J. Appl. Chem. 10, 1621–1626 (2001)
E.H. Mourid, M. Lakraimi, L. Benaziz, E.H. Elkhattabi, A. Legrouri, Wastewater treatment test by removal of the sulfamethoxazole antibiotic by a calcined layered double hydroxide. Appl. Clay Sci. 168, 87–95 (2019)
R. Kamaraj, A. Pandiarajan, M.R. Gandhi, A. Shibayama, S. Vasudevan, Eco–friendly and easily prepared graphene nanosheets for safe drinking water: removal of chlorophenoxyacetic acid herbicides. Chem. Select 2, 342–355 (2017)
S. Miyata, Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 31, 305–311 (1983)
M. Pavlovic, R. Huber, M. Adok-Sipiczki, C. Nardin, I. Szilagyi, Ion specific effects on the stability of layered double hydroxide colloids. Soft Matter 12, 4024 (2016)
T. Hibino, Anion selectivity of layered double hydroxides: effects of crystallinity and charge density. Eur. J. Inorg. Chem. 2018(6), 722–730 (2018)
S.N.M. Sharif, N. Hashim, I.M. Isa, S.B. Bakar, M.I. Saidin, M.S. Ahmad, M. Mamat, M.Z. Hussein, Controlled release formulation of zinc hydroxide nitrate intercalated with sodium dodecylsulphate and bispyribac anions: a novel herbicide nanocomposite for paddy cultivation. Arab. J. Chem. 13, 4513–4527 (2020)
H. Wu, H. Gao, Q. Yang, H. Zhang, D. Wang, W. Zhang, X. Yang, Removal of typical organic contaminants with a recyclable calcined chitosan-supported layered double hydroxide adsorbent: kinetics and equilibrium isotherms. J. Chem. Eng. Data 63, 159–168 (2018)
F.Z. Mahjoubi, A. Khalidi, A. Elhalil, B. Barka, Characteristics and mechanisms of methyl orange sorption onto Zn/Al layered double hydroxide intercalated by dodecyl sulfate anion. Sci. Afr. 6, e00216 (2019)
S. Boubakri, M.A. Djebbi, Z. Bouaziz, P. Namour, N. Jaffrezic-Renault, A.B.H. Amara, M. Trabelsi-Ayadi, I. Ghorbel-Abid, R. Kalfat, Removal of two anionic reactive textile dyes by adsorption into MgAl-layered double hydroxide in aqueous solutions. Environ. Sci. Pollut. Res. 25, 23817–23832 (2018)
Y. Seida, Y. Nakano, Removal of humic substances by layered double hydroxide containing iron. Water Res. 34(5), 1487–1494 (2000)
Z. Jia, S. Hao, X. Lu, Exfoliated Mg–Al–Fe layered double hydroxides/polyether sulfone mixed matrix membranes for adsorption of phosphate and fluoride from aqueous solutions. J. Environ. Sci. 70, 63–73 (2018)
F. Li, J. Liu, D.G. Evans, X. Duan, Stoichiometric synthesis of pure MFe2O4 (M = Mg Co, and Ni) spinel ferrites from tailored layered double hydroxide (hydrotalcite-like) precursors. Chem. Mater. 16, 1597–1602 (2004)
F. Li, L.H. Zhang, D.G. Evans, C. Forano, X. Duan, Structure and thermal evolution of Mg-Al layered double hydroxide containing interlayer organic glyphosate anions. Thermochim. Acta. 424(1–2), 15–23 (2004)
W.H. Wu, Q.P. Feng, M. Wang, G.W. Huang, Spherical Al-substituted ɑ-nickel hydroxide with high tapping density applied in Ni-MH battery. J. Power Sources 329, 170–178 (2016)
M. Laipan, J. Yu, R. Zhu, J. Zhu, A.T. Smith, H. He, D. O’Hare, L. Sun, Functionalized layered double hydroxides for innovative applications. Mater. Horiz. 7, 715–745 (2020)
Y. Ma, C. Shi, L. Lei, S. Sha, B. Zhou, Y. Liu, Y. Xiao, Research progress on polycarboxylate based superplasticizers with tolerance to clays—a review. Constr. Build Mater. 255, 119386 (2020)
M. Lakraimi, A. Legrouri, A. Barroug, A. De Roy, J.P. Besse, Preparation of a new stable hybrid material by chloride-2,4-dichlorophenoxyacetate ion exchange into the zinc–aluminium–chloride layered double hydroxide. J. Mater. Chem. 10, 1007–1011 (2000)
V. Prevot, C. Forano, J.P. Besse, Hybrid derivatives of layered double hydroxides. Appl. Clay Sci. 18, 3–15 (2001)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mourid, E.H., Lakraimi, M. & Legrouri, A. Removal and Release of the 2,4,5-Trichlorophenoxyacetic Acid Herbicide from Wastewater by Layered Double Hydroxides. J Inorg Organomet Polym 31, 2116–2128 (2021). https://doi.org/10.1007/s10904-020-01845-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01845-7