Abstract
Expanding a previous study of the immune response to SARS-CoV-2 in 10 New Jersey long-term care facilities (LTCFs) during the first wave of the pandemic, this study characterized the neutralizing antibody (NAb) response to infection and vaccination among residents and staff. Sera from the original study were tested using the semi-quantitative enzyme-linked immunosorbent cPass neutralization-antibody detection assay. Almost all residents (97.8%) and staff (98.1%) who were positive for IgG S antibody to the spike protein were positive for NAb. In non-vaccinated subjects with a history of infection (positive polymerase chain reaction (PCR) or antigen test), the distribution of mean intervals from infection to serology date was not significantly different for S antibody positives versus negatives. More than 80% of both were positive at 10 months. Similarly, the mean NAb titer for residents and staff was not associated with interval from PCR/antigen positive to serology date, F = 0.1.01, Pr > F = 0.4269 and F = 0.77, Pr > F = 0.6548 respectively. Titers remained high as the interval reached 10 months. In vaccinees who had no history of infection, the NAb titer was near the test maximum when the serum was drawn seven or more days after the second vaccine dose. In staff the mean NAb titer increased significantly as the vaccine number increased from one to two doses, F = 11.69, Pr > F < 0.0001. NAb titers to SARS-CoV-2 in residents and staff of LTCFs were consistently high 10 months after infection and after two doses of vaccine. Ongoing study is needed to determine whether this antibody provides protection as the virus continues to mutate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibody to the SARS-CoV-2 virus is one of the key components of the body’s protection against COVID-19. The receptor-binding domain (RBD) of the viral spike protein is a very precise target of IgG antibodies in SARS-CoV-2 patients [1, 2]. RBD-specific antibody levels have been shown to correlate with SARS-CoV-2 neutralizing antibodies in COVID-19 patients [3,4,5].
Limited data are available about duration of neutralizing antibody and IgG antibody to the spike protein on the viral surface. IgG antibody to the spike protein and neutralizing antibody titers peaked about three weeks after infection [6, 7] and then gradually decreased but persisted for more than three months [7]. IgG antibody to the spike protein has maintained for up to a year after infection in health care workers [8,9,10], for up to 210 days in long-term care facilities (LTCF) residents [11, 12], and in other subjects for up to one year [13,14,15,16]. In most [5, 17, 18] but not all studies [19], neutralizing antibody remained positive in asymptomatic health care workers (HCWs) and patients at least four months to a year post-infection and in both symptomatic and asymptomatic patients for a year or more after infection [5].
The GenScript cPass test is an enzyme-linked immunosorbent assay (ELISA) providing accurate detection of the neutralizing antibody against SARS-CoV-2 [20, 21]. As an ELISA, the test allows rapid testing of large numbers of specimens. The Rutgers New Jersey Medical School and the New Jersey State Department of Health previously reported the incidence of SARS-CoV-2 infection and the prevalence of IgG antibody to the nucleocapsid protein among residents and staff of 10 LTCFs in New Jersey in the first wave of the pandemic [22]. In this study we used this new neutralizing antibody test to further characterize this antibody response among residents and staff.
The objectives of this study were:
-
to measure the agreement between S protein antibody and an assay for neutralizing antibody to SARS-CoV-2, and
-
to describe the duration of that neutralizing antibody after infection and vaccination.
Methods
Sampling and Demographics
Residents and staff of 10 LTCFs in the state of New Jersey had provided informed consent and phlebotomy from November 2020 through March 2021 [22]. Subjects with remaining serum from the original study were tested for neutralizing antibodies against SARS-CoV-2 by the semi-quantitative cPass neutralization-antibody detection assay (GenScript, Piscataway, NJ).
Procedures and Serological Testing
A single serum specimen was available for each subject. The IgG antibody to the spike protein was performed using the VIDAS® SARS-CoV-2 IgG qualitative assay (Biomerieux, Cambridge, MA) (sensitivity 100%, 95% CI 88.3, 100, specificity 99.9%, 95% CI 99.4, 100) [23]. Total antibody to the nucleocapsid (N) protein (IgA, IgM, and IgG) testing was analyzed using the Bio-Rad Platelia SARS-CoV-2 Total Ab assay (sensitivity and specificity 98.0% (95% CI 89.5, 99.6) and 99.3% (95% CI 98.3, 99.7) [23]. Neutralizing antibody test was performed on all subjects who were S antibody positive using the cPass assay. This test has exhibited 96.1% (95 CI 94.9%, 97.3%) sensitivity at > 14 days post-positive real-time reverse transcription polymerase chain reaction (PCR) and 100% specificity (95% CI 98.0%, 100%) [24]. All three SARS-CoV-2 antibody tests were under FDA emergency use authorization (EUA). The N protein antibody was reported as positive, negative, or equivocal. S protein antibody was reported as positive or negative. The neutralizing antibody was reported as negative, detectable, or a numeric linear scale of 47 to greater than 185.
Factors Associated with Seropositivity
Duration of the antibody response was estimated as the difference between the date of the first positive SARS-CoV-2 PCR or protein antigen (PCR/antigen) test and the date of the serology. The interval was divided into 30-day periods (0–29 days, 30–59 days, up to 300–329 days). We contrasted the range of intervals comparing persons who were S antibody positive and S antibody negative.
COVID-19 or symptoms of a COVID-19-like illness were measured against the Centers for Disease Control and Prevention (CDC) clinical case definition [25].
The duration from vaccination date to serology date in individuals without evidence of prior infection was examined based on the most recent vaccine dose. Negative prior infection was defined as N antibody negative on serology and no history of a positive PCR/antigen test. No subject had more than the basic two doses of an mRNA vaccine.
The effect on mean neutralizing antibody was analyzed for the interval between PCR/antigen test result and serology, interval between vaccine one date and serology, and interval between vaccine two date and serology using the general linear model regression. Statistical analyses were performed using SAS software [26].
Results
There were 361 residents and 730 staff who consented to be in this study. Serology results were available on 337 residents and 667 staff. Absence of serology test was due to phlebotomy failure or unsatisfactory sample because of hemolysis, lipolysis or hyperlipidemia. Among residents, 129 had documentation in their medical record of a prior positive PCR or antigen test; (123 PCR positive/antigen negatives, 1 PCR negative/antigen positives, and 5 positives for both); among staff, 129 reported a prior positive test (115 PCR positive/antigen negatives, 7 PCR negative/antigen positives, and 7 positives on both). Date of positive test for residents ranged from March 27, 2020 to March 2, 2021, and for staff, from March 1, 2020 to February 1, 2021 (Fig. 1). Serologies were collected from November 8, 2020 to March 6, 2021 in residents and from October 20, 2020 to March 4, 2021 in staff.
Among those with a prior PCR/antigen positive result and no vaccination history prior to serology, 72 of 81 (89%) of residents and 84 of 101 (83%) of staff were S antibody positive. The mean interval between the first date of positive PCR/antigen result and the serology date was not significantly different by S antibody positive versus negative result (Fig. 2a and b). For nine residents with a prior positive PCR/antigen test, mean interval for S antibody negative residents was 215.3 days (95% CI 158.6, 272.0); the mean interval for the 72 positive residents with S antibody positive was 233.5 days (95% CI 221.6, 245.5) (F = 0.93, Pr > F = 0.3390). Mean interval for S antibody negative staff (n = 17) was 185.1 days (95% CI 141.4, 228.8) while the mean interval for positive staff (n = 84) was 203.6 days (95% CI 186.8, 220.5) (F = 0.78, Pr > F 0.3788). For both residents and staff, more than 80% of subjects were positive for S antibody consistently as the intervals extended to the maximum duration of 10 months.
Among those with an S antibody positive result, almost all were positive by the neutralizing test: 220 of 225 residents (97.8%) and 358 of 365 staff (98.1%).
In residents with a prior PCR/antigen positive result and a negative vaccination history prior to serology, the mean titer was 155.4, median 186, standard deviation 53.3. The mean neutralizing antibody titer was not associated with interval from PCR/antigen positive date to serology date (F = 0.1.01, Pr > F = 0.4269) (Fig. 3a). Gender (F = 0.35, Pr > F = 0.5539), age group (F = 0.93, Pr > F = 0.4297), and race/ethnicity (F = 0.93, Pr > F = 0.4547) were not associated with neutralizing antibody titer as well.
a Interval (from PCR/antigen to serology) versus mean neutralizing antibody titer, Residents n = 71. There is no upper error bar because there is no result beyond 186. There was no resident with an interval between 60 to 149 days. Median for 210–239, 240–269, and 270–299 days was 186. b Interval (from PCR/antigen to serology) versus mean neutralizing antibody titer, Staff, n = 84. Legend: ◊ mean ─ median ○ outlier. There is no upper error bar because there is no result beyond 186.. Median for 30–59 and 240–269 days was 186.
Similarly, in staff who tested positive for a prior PCR/antigen positive result and a negative vaccination history prior to serology, the mean was 135.3, median 151.5, standard deviation 53.6. The mean neutralizing titer was consistently high regardless of the interval (Fig. 3b). There was no significant association between titer by interval (F = 0,77, Pr > F = 0.6548), age group (F = 0.67, Pr > F = 0.6162), or race/ethnicity (F = 1.50, Pr > F = 0.2088). However, there was a higher mean titer among those with a history of symptoms meeting the Centers for Disease Control and Prevention case definition (case symptoms 143.6 versus not cases 115.9, F = 4.92, PrF > 0.0294), and by sex (female 149.9 versus male 125.4, F = 4.76, Pr > F = 0.0319). These relationships remained in a multiple regression modelling titer with interval, symptoms, and sex (respectively F = 0.86, Pr > F = 0.5775, F = 5.45, Pr > = 0.0224, F = 4.48, Pr > F = 0.0378).
Vaccination and Antibody Response
There were 12 residents who showed no evidence of SARS-CoV-2 infection (PCR/antigen negative and N antibody negative) and received only one dose of vaccine prior to serology. The interval between vaccination and serology date ranged from 5 to 33 days (8 residents had less than 14 days) and none of these were S antibody positive.
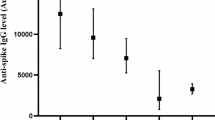
Among a randomly selected sample of 76 S antibody negative participants, 26 of 27 residents (96%) and 47 of 49 staff (96%) staff were also neutralizing test negative, respectively. There were 56 staff who showed no evidence of SARS-CoV-2 infection (PCR/antigen negative and N-antibody negative) and received only one dose of vaccine prior to serology. For staff with 1 to 13 days between vaccine dose one and serology, 6/35 (17%) were S antibody positive. Of those with 14 or more days between vaccine dose one and serology, 20/21 (95%) were S antibody positive (Fig. 4).
Using analysis of variance, staff with negative antibody had a mean interval of 6.9 days, whereas those with positive antibody had a mean interval of 19.2 days (F = 25.4, Pr > F < 0.0001).
Among the 26 staff who were S antibody positive, there was no significant difference of mean neutralizing titers by interval from single vaccine dose to serology date. The mean titer was 97.0, (95% CI 73.9, 120.1) and the median was 77.
Of the 16 residents who were tested after the second dose of vaccine, 12 were S antibody positive. Of 15 residents tested 7 or more days after vaccine dose two, 12 were S antibody positive. All 12 were near or at the maximum neutralizing antibody level (11 were > 185 and 1 was 185). Among the 42 staff with two doses of vaccine, 40 (95%) were S antibody positive; 7 of 8 tested 1–6 days after vaccine dose two and 33 of 34 tested 7 days or more after dose two were S antibody positive. Neutralization titer results were available for 40 S antibody positive staff who had two doses of vaccine. For these 40 staff, the mean titer by interval from vaccine dose two to serology date was 158.2 for interval 1–6 days (3), 182.2 for interval 7 to 13 days (23), and 181.9 for interval 14 days or more (14). There was no significant statistical different among neutralizing antibody level among those with 1–6, 7–13, or 14 or more days between vaccination dose two and serology (F = 0.64, Pr > F = 0.7803). The neutralization titer was generally at the maximum titer level if the tests were done 7 or more days after the second dose of vaccine. However, the mean neutralizing titers increased significantly as the mean moved from the intervals from vaccine one through vaccine two, F = 11.69, Pr > F < 0.0001, (Fig. 5).
Discussion
In this study of SARS-CoV-2 infection during the first wave of the pandemic, sera from LTCF residents and staff were measured for S protein antibody and neutralizing antibody titer at various intervals up to 10 months from initial infection. As far as we know, this is the only study in residents and staff in LTCFs that has documented high levels of neutralizing antibody at an interval of 10 months after infection. The cPass surrogate virus neutralization test offered neutralizing antibody test information applied to a large study sample. The duration of neutralization was consistent with duration reported in prior studies who documented this antibody up to a year after infection [5, 10, 18].
This finding is relevant because neutralizing antibody approximates the body’s immune response [5, 27]. Antibodies to the RBD of the spike protein and neutralizing antibodies are strongly correlated [3, 4, 17]. HCWs in Strasbourg, France who were positive for antibody to the RBD experienced a 96.7% reduction in new infections [10]. Spike antibody seropositive HCWs in Oxford, UK experienced no symptomatic infections and a sharp decrease in asymptomatic incidence in SARS-CoV-2 infection over 6 months of follow-up [28]. Residents in a Canadian LTCF who developed S protein antibody infected in a first outbreak were not infected in a second outbreak seven months later [12]. However, variants have selected for resistance to neutralizing antibodies [29, 30]. Combination vaccines inducing a neutralizing antibody response to both the first wave virus and to the Omicron variants have recently been announced [31].
In the current study, staff with symptoms meeting the Centers for Disease Control and Prevention case definition had a higher mean titer than those who did not report these symptoms. This is consistent with previous studies reporting a more robust immune response in those with symptoms versus no symptoms at the time of SARS-CoV-2 infection [5, 6].
Limitations
We did not have resources or the opportunity to obtain consecutive serologies on each participant. We created a composite picture by combining single serologies from many residents and staff with different intervals. This method lacked the precision provided when the interval is measured by sequential sera drawn on the same individual. However, our findings were consistent with researchers who found neutralizing antibody titers to be stable when measured in longitudinally-acquired sera.
Similarly, neutralizing antibodies were measured on only a single serology taken at various intervals from vaccine dose one or two to the date of serology. Neutralizing antibody titers were clearly higher in those with two versus one dose. The titer was near or at the maximum quickly after administration of vaccine dose two, and there was little difference in titer for serology drawn between interval of 1 to 6 days versus 14 days or more.
During the first wave of the pandemic, subjects may have had exposures to the virus in addition to the infection documented in our study. For the most part, our documentation of PCR/antigen testing was limited to the first positive date. Unreported exposures may have boosted the titer thereby increasing the longevity of antibody.
Conclusion
This study of residents and staff in LTCFs detected substantial neutralizing antibody titers to SARS-CoV-2 consistently 10 months after infection. Neutralizing antibody results from the cPass were consistent with the qualitative IgG spike protein antibody results but expanded findings to allow inferences into the duration of neutralizing antibody. Furthermore, titers at or near the maximum result were recorded following two vaccine doses. Further study is indicated to explore the level of protection these neutralizing antibodies provide as the virus continues to mutate.
Data Availability
All data and materials as well as software application or custom code support comply with field standards.
Code Availability
Code for the code for analysis can be made availability at the discretion of the authors. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Premkumar, P., Segovia-Chumbez, B., Jadi, R., et al. (2020). The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Science Immunology. https://doi.org/10.1126/sciimmunol.abc8413
Rodda, L. B., Netland, J., Shehata, L., Pruner, K. B., Morawski, P. A., Thouvenel, C. D., Takehara, K. K., Eggenberger, J., Hemann, E. A., Waterman, H. R., Fahning, M. L., Chen, Y., Hale, M., Rathe, J., Stokes, C., Wrenn, S., Fiala, B., Carter, L., Hamerman, J. A., King, N. P., et al. (2021). Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell, 184(1), 169-183.e17. https://doi.org/10.1016/j.cell.2020.11.029
Peterhoff, D., Glück, V., Vogel, M., et al. (2021). A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection, 49(1), 75–82. https://doi.org/10.1007/s15010-020-01503-7
Gallichotte, E. N., Nehring, M., Young, M. C., et al. (2021). Durable antibody responses in staff at two long-term care facilities, during and post SARS-CoV-2 outbreaks. Microbiology Spectrum, 9(1), e0022421. https://doi.org/10.1128/Spectrum.00224-21
Choe, P. G., Kang, C. K., Kim, K. H., et al. (2021). Persistence of neutralizing antibody response up to 1 year after asymptomatic or symptomatic SARS-CoV-2 infection. Journal of Infectious Diseases, 224(6), 1097–1099. https://doi.org/10.1093/infdis/jiab339
Long, Q. X., Tang, X. J., Shi, Q. L., et al. (2020). Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature Medicine, 26(8), 1200–1204. https://doi.org/10.1038/s41591-020-0965-6
Yamayoshi, S., Yasuhara, A., Ito, M., et al. (2021). Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine, 32, 100734. https://doi.org/10.1016/j.eclinm.2021.100734
Egbert, E. R., Xiao, S., Colantuoni, E., et al. (2021). Durability of spike immunoglobin G antibodies to SARS-CoV-2 among health care workers with prior infection. JAMA Network Open, 4(8), e2123256. https://doi.org/10.1001/jamanetworkopen.2021.23256
Lumley, S. F., Wei, J., O’Donnell, D., et al. (2021). The duration, dynamics, and determinants of severe acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clinical Infectious Diseases, 73(3), e699–e709.
Gallais, F., Gantner, P., Bruel, T., et al. (2021). Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. eBioMedicine, 71, 103561. https://doi.org/10.1016/j.ebiom.2021.103561
Carta, M., Bragagnolo, L., Tramarin, A., et al. (2020). Anti SARS-CoV-2 antibodies monitoring in a group of residents in a long term care facility during COVID-19 pandemic peak. Diagnosis (Berlin, Germany), 7(4), 395–400. https://doi.org/10.1515/dx-2020-0094
Tanunliong, G., Liu, A., Vijh, R., et al. (2022). Persistence of Anti-SARS-CoV-2 antibodies in long term care residents over seven months after two COVID-19 outbreaks. Frontiers in Immunology, 12, 775420. https://doi.org/10.3389/fimmu.2021.775420
Anand, S. P., Prévost, J., Nayrac, M., et al. (2021). Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Reports Medicine, 2(6), 100290. https://doi.org/10.1016/j.xcrm.2021.100290
Isho, B., Abe, K. T., Zuo, M., et al. (2020). Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Science Immunology, 5(52), 5511. https://doi.org/10.1126/sciimmunol.abe5511
Iyer, A. S., Jones, F. K., Nodoushani, A., et al. (2020). Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv: The preprint server for health sciences, 2020.07.18.20155374. https://doi.org/10.1101/2020.07.18.20155374
Masiá, M., Fernández-González, M., Telenti, G., et al. (2021). Durable antibody response one year after hospitalization for COVID-19: A longitudinal cohort study. Journal of Autoimmunity, 123, 102703. https://doi.org/10.1016/j.jaut.2021.102703
Wajnberg, A., Amanat, F., Firpo, A., et al. (2020). Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science, 370(6521), 1227–1230. https://doi.org/10.1126/science.abd7728
Havervall, S., Falk, A. J., Klingström, J., et al. (2021). SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. PLoS ONE, 17(1), e0262169.
Harrington, W. E., Trakhimets, O., & Andrade, D. (2021). Rapid decline of neutralizing antibodies is associated with decay of IgM in adults recovered from mild COVID-19. Cell Reports Medicine. https://doi.org/10.1016/j.xcrm.2021.100253
Taylor, S. C., Hurst, B., Charlton, C. L., et al. (2021). A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. Journal of Clinical Microbiology, 59(4), e02438-e2520. https://doi.org/10.1128/JCM.02438-20
Nandakumar, V., Profaizer, T., Lozier, B. K., et al. (2021). Evaluation of a surrogate enzyme-linked immunosorbent assay-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cPass neutralization antibody detection assay and correlation with immunoglobulin G commercial serology assays. Archives of Pathology and Laboratory Medicine, 145(10), 1212–1220. https://doi.org/10.5858/arpa.2021-0213-SA
Friedman, S. M., Davidow, A. L., Gurumurthy, M., et al. (2022). Antibody seroprevalence, infection and surveillance for SARS-CoV-2 in residents and staff of new jersey long-term care facilities. Journal of Community Health. https://doi.org/10.1007/s10900-022-01104-5
https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021
SAS 9.4 (SAS Institute)
Khoury, D. S., Cromer, D., Reynaldi, A., et al. (2021). Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nature Medicine, 27(7), 1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Lumley, S. F., O’Donnell, D., Stoesser, N. E., et al. (2021). Antibody status and incidence of SARS-CoV-2 infection in health care workers. New England Journal of Medicine, 384(6), 533–540. https://doi.org/10.1056/NEJMoa2034545
Weisblum, Y., Schmidt, F., Zhang, F., et al. (2020). Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife, 9, e61312. https://doi.org/10.7554/eLife.61312
Cao, Y., Wang, J., Jian, F., et al. (2022). Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature, 602(7898), 657–663. https://doi.org/10.1038/s41586-021-04385-3
Chalkias, S., Harper, C., Vrbicky, K., et al. (2022). A bivalent Omicron-containing booster vaccine against Covid-19. medRxiv, 06.24.22276703. https://doi.org/10.1101/2022.06.24.22276703
Acknowledgements
Ms. Dana Woell contributed to the laboratory management. Mr. Stephen Modica directed the references. The Rutgers University Biostatistics and Epidemiology Services (RUBIES) team provided statistical support.
Funding
This work was supported by a grant from the New Jersey Department of Health to the Rutgers New Jersey Medical School.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by SF. Subsequent versions were edited by SF, JL, PT, EL and MG with review by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
All authors agreed with the study content and all gave explicit consent to Rutgers NJ Medical School where the work was carried out.
Ethical Approval
The authors state that they have approval from the Rutgers New Jersey Medical School Institutional Review Board. Consent to participate was signed by all study subjects.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Friedman, S.M., Li, J., Thomas, P. et al. Neutralizing Antibody Responses Among Residents and Staff of Long-Term Care Facilities in the State of New Jersey During the First Wave of the COVID-19 Pandemic. J Community Health 48, 50–58 (2023). https://doi.org/10.1007/s10900-022-01142-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-022-01142-z