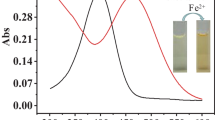
Abstract
In order to better monitor the content of Fe3+ and H2S in the biological environment, two new fluorescent probes were designed and synthesized. With the addition of Fe3+, the strong fluorescence emission of two probes was significantly quenched due to the paramagnetic effect of Fe3+. With the further addition of S2−, the fluorescence intensity was quickly restored. Two probes showed high selectivity and strong sensitivity for the detection of Fe3+ and S2−, and the fluorescence intensity “ON-OFF-ON” was accompanied with the interaction process. At the same time, two probes displayed good anti-interference ability which was not interfered by the existence of other ions. In addition, two probes illustrated fast response time to Fe3+, S2− and small cytotoxicity to cells. Therefore, two probes can provide a potential ideal tool for detecting Fe3+ and H2S in organisms and the environment.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author.
References
Danilewicz JC (2003) Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: central role of iron and copper. Am J Enol Viticult 54:73–85. https://doi.org/10.1016/S0304-4238(02)00148-6
Zhang YP, Li XF, Yang YS et al (2021) A novel fluorescent probe based on pyrazole-pyrazoline for Fe (III) ions recognition. J Fluoresc 31:29–38. https://doi.org/10.1007/s10895-020-02632-w
Geng YR, Chen LP, Wan QL et al (2021) A novel [1,2,4] triazolo [1,5-a] pyrimidine derivative as a fluorescence probe for specific detection of Fe3+ ions and application in cell imaging. Anal Chim Acta 1187:339168. https://doi.org/10.1016/j.aca.2021.339168
Li LJ, Wang JH, Xu SH et al (2022) Recent progress in fluorescent probes for metal ion detection. Front Chem 10:875241. https://doi.org/10.3389/fchem.2022.875241
Nayab PS, Shkir M, Gull P et al (2017) A highly sensitive “OFF-ON” optical and fluorescent chemodosimeter for detecting iron (III) and its application in practical samples: an investigation of Fe3+ induced oxidation by mass spectrometry. J Photoch Photobio A 347:209–217. https://doi.org/10.1016/j.jphotochem.2017.07.048
Hijji YM, Rajan R, Shraim AM (2022) 3-Aminopyridine salicylidene: a sensitive and selective chemosensor for the detection of Cu(II), Al(III), and Fe(III) with application to real samples. Int J Mol Sci 23:13113. https://doi.org/10.3390/ijms232113113
Chang D, Zhao ZH, Niu WJ et al (2021) Iron ion sensing and in vitro and in vivo imaging based on bright blue-fluorescent carbon dots. Spectrochim Acta A 260:119964. https://doi.org/10.1016/j.saa.2021.119964
Deng HJ, Tian C, Gao ZW et al (2020) Highly luminescent N-doped carbon dots as a fluorescence detecting platform for Fe3+ in solutions and living cells. Analyst 145:4931–4936. https://doi.org/10.1039/D0AN00208A
Üçüncü M (2023) A phenalenone-based fluorescent probe for the detection of Fe3+ ions. J Fluoresc 33:707–712. https://doi.org/10.1007/s10895-022-03117-8
Hofmann J, Watson V, Scharaw B (2015) Groundwater quality under stress: contaminants in the Kharaa River basin (Mongolia). Environ Earth Sci 73:629–648. https://doi.org/10.1007/s12665-014-3148-2
Aroca A, Gotor C, Bassham DC et al (2020) Hydrogen sulfide: from a toxic molecule to a key molecule of cell life. Antioxidants 9:621. https://doi.org/10.3390/antiox9070621
Liu LQ, Qu HM, Li XM et al (2021) Novel naphthalimide derived fluorescent probe based on aggregation-induced emission for turn-on detection of hydrogen sulfide. Tetrahedron 81:131923. https://doi.org/10.1016/j.tet.2021.131923
Li J, Su Z, Yu CM et al (2021) Recent progress in the development of sensing systems for in vivo detection of biological hydrogen sulfide. Dyes Pigm 192:109451. https://doi.org/10.1016/j.dyepig.2021.109451
Shu W, Zang SP, Wang C et al (2020) An endoplasmic reticulum-targeted ratiometric fluorescent probe for the sensing of hydrogen sulfide in living cells and zebrafish. Anal Chem 92:9982–9988. https://doi.org/10.1021/acs.analchem.0c01623
Zhong KL, Hu XL, Zhou SY et al (2021) Mitochondria-targeted red-emission fluorescent probe for ultrafast detection of H2S in food and its bioimaging application. J Agr Food Chem 69:4628–4634. https://doi.org/10.1021/acs.jafc.1c00862
Wang DM, He JJ, Sun JY (2021) Two-photon ratiometric fluorescent probe based on NBD-amine functionalized semiconducting polymer nanoparticles for real-time imaging of hydrogen sulfide in living cells and zebrafish. Talanta 228:122269. https://doi.org/10.1016/j.talanta.2021.122269
Zhu MQ, Sun L, Liu XN et al (2023) A reversible CHEF-based NIR fluorescent probe for sensing Hg2+ and its multiple application in environmental media and biological systems. Sci Total Environ 874:162460. https://doi.org/10.1016/j.scitotenv.2023.162460
Gao ZG, Zhang L, Liu HB et al (2022) A novel rhodol-based fluorescence turn-on probe for selective hydrogen sulfide detection in environment water and living cells. J Photoch Photobio A 423:113598. https://doi.org/10.1016/j.jphotochem.2021.113598
Jothi D, Munusamy S, Iyer SK (2021) A new sensitive “turn-on” fluorescent probe based on naphthalimide: application in visual recognition of hydrogen sulfide in environmental samples and living cells. J Photoch Photobio A 420:113491. https://doi.org/10.1016/j.jphotochem.2021.113491
Zhou RQ, Cui GL, Qi QR et al (2020) The synthesis and bioimaging of a biocompatible hydrogen sulfide fluorescent probe with high sensitivity and selectivity. Analyst 145:2305–2310. https://doi.org/10.1039/C9AN02323B
Shen W, Wang P, Xie ZD et al (2021) A bifunctional probe reveals increased viscosity and hydrogen sulfide in zebra fish model of Parkinson’s disease. Talanta 234:122621. https://doi.org/10.1016/j.talanta.2021.122621
Chen SL, Huang YJ, Yang Y et al (2021) Ultrasensitive Fe3+ ion detection based on pH-insensitive fluorescent graphene nanosensors in strong acid and neutral media. New J Chem 45:5829–5836. https://doi.org/10.1039/D0NJ06201D
Gouda AA (2016) A new coprecipitation method without carrier element for separation and preconcentration of some metal ions at trace levels in water and food samples. Talanta 146:435–441. https://doi.org/10.1016/j.talanta.2015.09.005
Li T, Shang DW, Gao SW et al (2022) Two-dimensional material-based electrochemical sensors/biosensors for food safety and biomolecular detection. Biosens (Basel) 12:314. https://doi.org/10.3390/bios12050314
Xie ZC, Sun XF, Jiao JM et al (2017) Ionic liquid-functionalized carbon quantum dots as fluorescent probes for sensitive and selective detection of iron ion and ascorbic acid. Colloid Surf A 529:38–44. https://doi.org/10.1016/j.colsurfa.2017.05.069
Hall JR, Schoenfisch MH (2018) Direct electrochemical sensing of hydrogen sulfide without sulfur poisoning. Anal Chem 90:5194–5200. https://doi.org/10.1021/acs.analchem.7b05421
Luo YN, Zhu CZ, Du D et al (2019) A review of optical probes based on nanomaterials for the detection of hydrogen sulfide in biosystems. Anal Chim Acta 1061:1–12. https://doi.org/10.1016/j.aca.2019.02.045
Su DD, Cheng D, Lv Y et al (2020) A unique off-on near-infrared QCy7-derived probe for selective detection and imaging of hydrogen sulfide in cells and in vivo. Spectrochim Acta A 226:117635. https://doi.org/10.1016/j.saa.2019.117635
Zhang XQ, Jin XD, Zhang CT et al (2021) A fluorescence turn-on probe for hydrogen sulfide and biothiols based on PET & TICT and its imaging in HeLa cells. Spectrochim Acta A 244:118839. https://doi.org/10.1016/j.saa.2020.118839
Du YT, Wang HL, Zhang T et al (2022) An ESIPT-based fluorescent probe with fast-response for detection of hydrogen sulfide in mitochondria. Spectrochim Acta A 265:120390. https://doi.org/10.1016/j.saa.2021.120390
Sureshkumar K, Ramakrishnappa T (2022) A near infrared fluorescent probe for naked eye visualization and selective detection of hydrogen sulfide. Mater Today: Proc 49:597–602. https://doi.org/10.1016/j.matpr.2021.04.512
Zhang XY, Li Y, Wang YY et al (2022) Nitrogen and sulfur co-doped carbon dots with bright fluorescence for intracellular detection of iron ion and thiol. J Colloid Interf Sci 611:255–264. https://doi.org/10.1016/j.jcis.2021.12.069
Kim JK, Bong SY, Park R et al (2022) An ESIPT-based fluorescent turn-on probe with isothiocyanate for detecting hydrogen sulfide in environmental and biological systems. Spectrochim Acta A 278:121333. https://doi.org/10.1016/j.saa.2022.121333
Yan L, Gu QS, Jiang WL et al (2022) Near-infrared fluorescent probe with large stokes shift for imaging of hydrogen sulfide in tumor-bearing mice. Anal Chem 94:5514–5520. https://doi.org/10.1021/acs.analchem.1c04169
Yang WY, Anusuyadevi K, Lu PH et al (2022) A two photon fluorescent probe for highly selective detection and endogenous imaging of hydrogen sulfide. Spectrochim Acta A 273:121043. https://doi.org/10.1016/j.saa.2022.121043
Pang XH, Sun Lon, Wan J et al (2023) New insights into the binding mechanism of lysozyme by 2-sulfanilamido-4-methylpyrimidine and 3-sulfanilamido-5-methylisoxazole: density function theory, multispectral techniques and molecular docking. J Lumin 255:119559. https://doi.org/10.1016/j.jlumin.2022.119559
Zhu MQ, Pang XH, Wan J et al (2022) Potential toxic effects of sulfonamides antibiotics: molecular modeling, multiple-spectroscopy techniques and density functional theory calculations. Ecotox Environ Safe 243:113979. https://doi.org/10.1016/j.ecoenv.2022.113979
Zhu MQ, Pang XH, Wang KQ et al (2023) Enantioselective effect of chiral prothioconazole on the conformation of bovine serum albumin. Int J Biol Macromol 240:124541. https://doi.org/10.1016/j.ijbiomac.2023.124541
Zhu MQ, Zhao ZY, Liu XN et al (2021) A novel near-infrared fluorimetric method for point-of-care monitoring of Fe2+ and its application in bioimaging. J Hazard Mater 406:124767. https://doi.org/10.1016/j.jhazmat.2020.124767
Lignell M, Becker HC (2010) Recognition and binding of a helix-loop-helix peptide to carbonic anhydrase occurs via partly folded intermediate structures. Biophys J 98:425–433. https://doi.org/10.1016/j.bpj.2009.10.038
Nie F, Lu JR (2007) Determination of ketotifen by using calcein as chemiluminescence reagent. Anal Chim Acta 592:168–172. https://doi.org/10.1016/j.aca.2007.04.041
Lee C, Yang WT, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/physrevb.37.785
Funding
This work was supported by Henan Provincial Science and Technology Research Project, China (222102230027). Author Lixia Liu has received research support from Henan Province, China.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Bingqing Liu, Lixia Liu, Jia Wang and Yingling Wang. The first draft of the manuscript was written by Xuefang Shang and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable, because this study does not involve animal manipulation.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to Participate
All authors gave their consent to participate in the research.
Consent for Publication
All authors gave their consent to participate in the publication of the research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shang, X., Liu, B., Liu, L. et al. Difunctional Fluorescent Probes for Iron and Hydrogen Sulfide Detection Based on Diphenyl Derivative. J Fluoresc 34, 1269–1278 (2024). https://doi.org/10.1007/s10895-023-03374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03374-1