Abstract
In this review we will present several research papers pertaining to white colour (or other) emission from Dy3+ doped and undoped phosphor materials. The search for a single component phosphor material that could deliver high quality white light under UV or near UV excitation is an area of active research for commercial purposes. Amongst all rare earth elements Dy3+ is the only ion that could deliver simultaneously blue and yellow light under UV excitation. In optimizing the Yellow/Blue emission intensity ratios, white light emission can be realized. Dy3+ (4f9) displays approximately 4 emission peaks at around 480 nm, 575 nm, 670 and 758 nm corresponding to transitions from the metastable 4F9/2 state to various lower states, such as 6H15/2 (blue), 6H13/2 (yellow), 6H11/2 (red) and 6H9/2 (brownish red), respectively. In general, the hypersensitive transition at 6H13/2 (yellow) is electric dipole in nature and becomes prominent only when Dy3+ ions are positioned at low symmetric sites with no inversion symmetry in the host matrix. On the other hand, the blue magnetic dipole transition at 6H15/2 becomes prominent only when Dy3+ ions are positioned at highly symmetric sites in the host material with inversion symmetry. Despite the white colour emission from the Dy3+ ions, these transitions are mainly associated with parity forbidden 4f -4f transitions, the white light produced maybe diminished at times, hence the need to include a sensitizer to bolster the forbidden transitions experienced by Dy3+ ions. In this review we will focus on the variability of the Yellow/Blue emission intensities in different host materials (phosphates, silicates, and aluminates) from Dy3+ ions (doped or undoped) by studying their photoluminescent properties (PL), their CIE chromaticity coordinates and correlated colour temperature (CCT) values for white colour emissions that is adaptable to different environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White light-emitting diodes (WLED) is the next generation of solid-state lighting (present day use) that has replaced the traditional incandescent and fluorescent lamps of bygone days [1, 2]. The reason for its use is because of its superior brightness, long lifetimes, absence of toxic mercury and cost saving energy properties [1, 2]. There are essentially three ways to fabricate WLEDs: (i) a combination of a blend of red, blue, and green phosphor materials, (ii) a dual combination of a blue emitting light diode with a yellow emitting phosphor material, where the most famous Ce3+ doped yttrium aluminum garnet (YAG: Ce3+) commercial phosphor material comes to mind, and finally (iii) fabrication of single phased all inclusive phosphor material doped with specific rare-earth ions for colour tuning [1, 3]. From case (ii) above, a similar combination of colours to obtain white light can be achieved by doping Dy3+ ion in a single phased host material, because the characteristic emission colour for this Dy3+ ion lies in the blue and yellow colour regions of the electromagnetic spectrum.
Trivalent dysprosium Dy3+ (4f9) generally possesses 2 intense characteristic emission peaks at 485 nm, which corresponds to a blue magnetic dipole 4F9/2 → 6H13/2 transition and usually, a hypersensitive yellow electric dipole 4F9/2 → 6H15/2 transition at 575 nm, respectively. The optimal yellow to blue (Y/B) intensity peak emission ratios yields white light. The position of the Dy3+ ions in the host environment is of crucial importance for efficient white light emissions. When Dy3+ ions are positioned at low symmetric sites in the environment of the host material with no inversion center, yellow emissions at 485 nm are of relatively higher intensity compared to blue light emissions at 575 nm wavelength are observed. On the other hand, when Dy3+ ions are positioned at highly symmetric sites in the environment of the host without inversion symmetry, then blue light colour emissions are relatively higher than the yellow light colour emissions.
A host on its own by its very nature is unable to emit white light, thus the necessity for the embedding of dopant ions, which in this case is Dy3+ ions [1]. The search for a single phased phosphor material for white light emission for LED application when doped with Dy3+ ions or co-doped with other rare-earth ions (other colours such as blue and green) for persistent luminescence under UV excitation is an area of active research. There is potential need to fabricate materials with unconventional emission properties, where previously the existence of defects was found to suppress the photoluminescence properties of materials, but now defects are found to be a storage or a trapping site for charges in the generation of persistence luminescence that was observed with specific host materials [3]. Potential host materials that could fit this criterion could be phosphates, silicates, aluminates, borates, etc. However, white light emissions from Dy3+ doped host materials suffer some drawbacks due its parity forbidden nature of its 4f – 4f transitions of the Dy3+ ions.
In the later part of this review, an attempt is made to ameliorate this situation by co-doping it with other rare –earth ion to bolster the parity forbidden nature of the Dy3+ ions. However, this process may lead to different colour emissions (besides white colour light), depending on whether the dopant (Dy3+) is a sensitizer or an activator, but the result is for prolonged luminescence emissions long after the UV excitation source is removed.
In this review, we will focus on research papers, firstly for enhancing white light emissions through Dy3+ doping, and in the second part, a look at persistent luminescence when Dy3+ is co-doped with other rare-earth ions in different host materials, as well as the mechanism for persistence luminescence will be discussed.
Factors for Consideration for White Light Efficiency and Persistent Luminescence in Singly doped Dy3+ and Co-Doped Host Materials
Researchers in this review have published many papers using Dy3+ ions for white light emissions or co-doped it for persistent luminescence, but have not reached the desired standards for commercialization, due to degradation of the light or lack of consistency for prolonged luminescence (or the inefficiency of the mechanism).
Some, if not all these factors need to be factored in producing optimal emission characteristics (white light efficiency in the first instance and consistency of its illumination in the second instance) [4, 5]:
-
(a)
Low preparation costs.
-
(b)
High CRI and low CCT values.
-
(c)
High chemical and thermal stabilities.
-
(d)
Appropriate choice of host compounds and dopants.
-
(e)
Nature of Y/B intensity ratios in producing electric dipole or magnetic dipole transitions.
-
(f)
Emission lifetimes: fluorescence or phosphorescence emissions.
-
(g)
Influence of concentration on the Y/B ratio in Dy3+ doped materials.
-
(h)
Mechanism of white light colour emission from Dy3+ doped host materials.
Low Preparation Costs
In advancing the use white LEDs or materials for prolonged luminescence, facile methods of preparation at low cost are necessary. Typical methods of preparation at a relatively low cost used by many researchers are the solid-state reaction and the combustion methods of synthesis. Single phased phosphor materials as opposed to the use of tricolor phosphor materials is a cost saving procedure in producing desired phosphor materials. In the case of aluminates and silicates, synthesis method play an important role in persistent luminescence, therefore a variety of synthesis methods were employed.
High CRI and Low CCT Values
According to Wikipedia [November 2022], the colour rendering index (CRI) is “a quantitative measure of the ability of a light source to reveal the colours of various objects faithfully in comparison with a natural or standard light source”. This means that a light source must depict the natural colour of an object [4]. CRI values range from 0 to 100, thus a value of 100 implies that a light source is identical to the spectrum of daylight. In general, light sources should have CRI values greater than 80 for it to be commercially acceptable. The other factor for consideration is the correlated colour temperature (CCT) appearance. This is defined as the temperature appearance of a white LED light source (lamp). It is measured in kelvins and is determined using McCamy’s empirical formula: CCT = -449n3 + 3525n2 -6823n + 5520.33, where n represents the inverse slope line and is given by n = (x – 0.332)/ (y – 0.186). In general, CCT values fall in 4 categories: (2700 – 3000 K) is regarded as warm white light, (3000 – 4500 K) is regarded as bright white light and (4500 – 6500 K) is regarded as natural daylight and colour colours beyond these values are regarded as coolish bluish white light [Wikipedia, November 2022].
High Thermal and Chemical Stabilities
Beside the physical properties of these materials (CRI, CCT and luminescence efficiencies) mentioned above, white light emitting or persistent luminescent phosphors, need to be chemically and thermally stable. This means that they need to withstand temperature chemical changes. Phosphor materials having such characteristics are phosphates, silicates, and aluminates.
Appropriate Choice of Host Compounds and Dopants
Host materials by their very nature do not produce light emissions, hence the need to choose a dopant or co-dopant that could act as luminescent centers in the host material. Shrivastava et al. [4] has identified several host materials that could be doped with Dy3+ ions for white colour emissions. Silicate and aluminate host compounds appear to take the lead in this regard because much of their properties relate to prolonged emissions long after the removal of the UV excitation source. Other researchers have focused on phosphates as host materials for the embedding of the Dy3+ ion dopant to produce enhanced white colour luminescence. Besides white colour emissions produced from Dy3+ doped host materials, other properties such as persistent luminescence has also been observed when Dy3+ ion is co-doped with other rare earth ions. Aluminates has gained much popularity as a host material because of its high chemical stability, high quantum yield, as well as a wide band gap of around 6 eV [6]. Silicates, likewise, have similar properties; they have a high chemical stability, a high quantum yield and have a wide band gap and is the most widely used host material for persistence luminescence [6]. On the other hand, phosphates have a relatively large band gap, possess different crystallographic structures, and display excellent luminescence properties but have lower persistent luminescence property compared to silicates and aluminates. These host materials show enhanced long afterglow effects when codoped with Eu2+ ions. Besides using Dy3+ as a dopant, others have considered Eu3+, Sm3+, Ce3+ as compatible dopant pairs in the above-mentioned host materials. Single-phased host materials are important because they are cost effective in their fabrication as compared to using 2 or 3 phosphor materials to produce the same effect.
Nature of Y/B Intensity Ratios in Producing Electric Dipole (ED) or Magnetic Dipole (MD) Transitions
It is noted that some Dy3+ doped phosphor materials experience either electric dipole or magnetic dipole transitions. When yellow colour emissions (ED) are higher than the blue colour emissions (MD), then the intensity Y/B ratio is greater than 1, but if the blue colour emission is higher than the yellow emissions, then the peak intensity Y/B emission ratio is less than 1.
Emission Lifetimes: Fluorescence or Phosphorescence Emissions
Fluorescence electronic transitions happens almost instantaneously (t < 10− 8s) without changes in its spin state, but phosphorescence emissions are much slower (t > 10− 8s) and is accompanied by changes in its electronic spin states [7]. Extensive research is being done to find Dy3+ doped or co-doped phosphor materials that exhibits persistent luminescence long after the excitation source is removed. In this regard silicates and aluminates are promising candidates for such endeavors.
Influence of Concentration on the Y/B Ratio in Dy3+ Doped Materials
If Dy3+ ion has the same valency as the cation (e.g. Y3+), then the intensity ratio Y/B will hardly change with increases in concentration. On the other hand, if Dy3+ ion has a different valency than the cation (e.g. Y2+) then the Y/B intensity ratio will change with increases in concentration [5] but not so with temperature changes.
Mechanism of White Light Colour Emission from Dy3+ Doped Host Materials
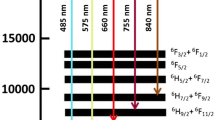
The energy level diagram below is taken from the paper of Shamal et al. [8], shows the energy transfer mechanism of a typical Dy3+ doped host material. This will typically happen when Dy3+ is doped with aluminates, phosphate, or silicate host materials, since the ion will be positioned within the band gap of these materials. It should be noted that either the blue light or yellow light emission could be more prominent dependent on whether Dy3+ ions are positions within the environment with inversion or without inversion symmetry, thus affecting the nature of the white light produced.
Initially, Dy3+ ion is excited using an excitation wavelength of 447 nm. The excited electron moves from the ground state (6H15/2) of Dy3+ ion to its upper excited state (4I15/2). It then relaxes non-radiatively to the metastable 4F9/2 state, populating it and thereafter makes various downward transitions, resulting in different colour emissions. The emission at wavelength 484 nm corresponds to blue light (4F9/2 → 6H15/2), a yellow colour emission occurs at 576 nm (4F9/2 → 6H15/2), a red colour emission occurs at 664 nm (4F9/2 → 6H11/2) and finally a brown colour emission occurs at 754 nm (4F9/2 → 6H9/2, 6F11/2). Appropriate ratios of Yellow to Blue will result in white colour light. When Dy3+ is codoped with other rare earth ions, the mechanism of such is discussed at a later stage in this paper
White light Emissions from Singly Doped Dy3+ Ions Corresponding to Magnetic or Electric Dipole Transitions from Phosphate Type Materials
Several research papers [9,10,11,12,13,14,15] have been published with Dy3+ as a dopant ion, corresponding to magnetic dipole transitions, as shown in Table 1(a). For all these papers the common synthesis method is the solid-state reaction method. The excitation wavelength for all these phosphor materials falls in the UV range from 348 to 387 nm. The PL peak intensities, corresponding to blue emissions is situated in the narrow wavelength range of 480 to 487 nm, while that for yellow PL emissions were found in the 571 to 577 nm wavelength range. As far as the Y/B intensity ratio is concerned, they are all less than 1, implying that the blue light emissions dominate over the yellow colour emissions. This further implies that Dy3+ ions are located at high symmetry sites with inversion center in the environment surrounding the phosphate host material [12]. The CCT values calculated for these Dy3+ doped phosphate materials fall in the cool bluish white colour range, with temperature values in the range from 5962 to 12,606 K. Phosphates of this nature are mainly adaptable for outdoor settings. In all cases, the Y/B values appear to change with concentrations, implying that Dy3+ ions are displaced by ions of different valences (Sr2+, Ca2+, Na+), leading to the creation of defects in the crystal lattice.
Besides the magnetic dipole transitions observed from singly Dy3+ doped phosphate materials, Table 1(b) also displays electric dipole transitions associated with these materials [16,17,18,19,20]. Likewise, the solid- state reaction method was the common method of synthesis of these materials. The excitation wavelength for these materials is a wide range from 325 to 410 nm. The PL emission peak for the less dominant blue colour ranged from 480 to 496 nm, while the dominant yellow colour peak ranged from 573 to 576 nm. The dominant hypersensitive yellow colour emissions is due to Dy3+ ions being located at low symmetry sites without inversion. The CIE coordinates, which was determined from the PL intensity peaks, was found to produce CCT values in the range from 3707 to 6799 K, which is typically in the warm to cool blue to white colour regions. This range of temperatures is ideal for energetic environmental settings, promoting alertness and sharpness in various indoor or outdoor activities. The intensity Y/B ratios in all cases is greater than 1 for electric dipole transitions and whose values changes with concentrations, implying that Dy3+ ions are displaced by ions of different valences (Ba2+, K+, Na+, Ca2+), thereby creating defects in the host matrix. The warm white light produced in most of this phosphate doped materials could be considered for commercialization when excited by UV light.
White Light Emissions from Co-Doped Dy3+ Ions Corresponding to Magnetic or Electric Dipole Transitions from Phosphate Type Materials
In the case of co-doped Dy3+ phosphate host materials, the mechanism of white light emission is different from that of singly doped phosphor materials, as seen in Table 1(c) [21, 22]. The difference emanates from type of co-dopant used and the role it plays, either as an activator or a sensitizer in the host compound. If Ce3+ ion is used as a dopant, it can effectively enhance the luminescence characteristics of the phosphate material, because of it has a broad and intense emission band due to its parity and spin allowed 4f -5d transition and which is electric dipole in nature [23]. The effect of this is to boost the parity forbidden nature of the Dy3+ transitions [24]. On the other hand, the addition of Sm3+ as a co-dopant will play a crucial role in addressing the shortage of the red ingredient of white light [21]. One can say that the white light emission from singly doped Dy3+ ions have drawbacks in producing light emissions with a low CRI and a high CCT value [21]. Thus, Sm3+ as a co-dopant can ameliorate this situation by producing high quality white light of a high CRI and a low CCT value. Further, the addition of Eu3+ as a co-dopant, exhibits strong red colour emissions and is a useful ingredient for high quality white light production. In this regard it can be said that the addition of Eu3+ as a co-dopant is effective in producing better warm colour white light [22]. In the case of co-doped xDy3+ /yEu3+ (where x = 3 mol % and y = 2, 4, 6, 8 and 10 mol%) NaSrPO4 phosphor material, the white light produced will vary for each pair of CIE coordinates, as shown in Table 1(c) [22]. For this example, the intensity of the Dy3+ ion is kept constant at 3 mol% while the concentrations of Eu3+ is varied from 2 mol% to 10 mol%. From the PL emission spectra, it is observed that the blue and yellow emissions of Dy3+ ions decrease for increases in the concentration of Eu3+ ions. This implies that energy is being effectively transferred from Dy3+ ions to Eu3+ ions. The CIE as well as the CCT values are shown in Table 1(c) for such variations.
From the above table, the maximum PL emission occurs for the phosphor material having CIE coordinates (0.295, 0.330) yet its CCT value is high and is not ideal for commercialization, but with concentration tuning its CIE and CCT values was adjusted to a warm white light, as can be seen in Table 1 (c). Thus, co-doping phosphate host materials can be an effective way of tuning light emission to the desired white colour light as compared to singly doped Dy3+ ion. As with singly doped Dy3+ ions, the excitation and emission wavelengths are in a similar range. For these samples, blue light emission was more dominant than the yellow colour emission and its CCT values were tuned with concentration variations. The preparation methods for these samples varied from hydrothermal to sol-gel methods of synthesis. Whilst these two samples provide compelling reasons for co-doping and tuning with concentration, other researchers have focused on optimizing the x and y values when obtaining their CIE and CCT values [23, 24], as seen in Table 1(d). When Ca3Mg3(PO4)4 is doped with Dy3+ ions (Li+ is a compensator ion) only, the CIE value at optimum concentration is (0.265, 0.339), which corresponds to a CCT value of 9536 K. Further, in co-doping Ca3Mg3(PO4)4 with Ce3+ ions, with Ce3+ ion concentration being fixed while the concentration of Dy3+ was varied, then at optimal values of these concentrations, their CIE value changed to (0.317, 0. 393), corresponding to a CCT value of 4739 K. In co-doping, we observe 2 fundamental changes; in the first case, we observe magnetic dipole transition, and secondly when co-doped with Ce3+ ions, the transition changed from magnetic dipole to electric dipole transition. In this scenario the Y/B value changed from 0.9 to 1.265 brought about by co-doping the material with Ce3+ ions. Further the cool white colour produced results from the energy transfer from Ce3+ ion to Dy3+ ions. Combustion synthesis method was used here and besides the two typical emission wavelengths for blue and yellow lights, the excitation wavelength for this sample was at 316 nm.
Similarly, in co-doping Zn2P2O7 with Ce3+ ions have produced enhanced white emissions in the bluish region due to the energy transfer from Ce3+ ions to Dy3+ ions. This transition is due to the magnetic dipole interactions between the Dy3+ ions. At optimal concentration of Ce3+ (x fixed) and Dy3+ (y varied) ions, the CIE value obtained was (0.2764, 0.2876), which corresponds to a CCT value of 10,490 K, which is a coolish blue, white light. The excitation wavelength applied is a deep UV-B 290 nm wavelength, where the solid- state reaction method was used for preparing these samples.
White Light Emissions from Singly Doped Dy3+ Ions with Silicates as Host Materials
The next category of host materials for consideration in white light emission is through doping Dy3+ ions with silicates. Silicates are easy to prepare and have excellent thermal and chemical stability [25]. The role of silicates in this scenario is for the generation of phosphorescence white light (t > 10− 8s) instead of fluorescence white light (t < 10− 8s) [4]. When Dy3+ ions are embedded in silicate host materials, it creates traps, which prolongs the emission of white light due to the continuous energy transfer from these traps to luminescent centers [4]. Several research papers [26,27,28,29,30] have been considered in respect of singly doped Dy3+ silicate materials, as seen in Table 2(a). The most popular synthesis route is the solid-state reaction method, followed by the combustion synthesis route of preparation. The excitation wavelengths have a wide range from 314 to 352 nm.
Typical emissions bands in the blue (470–480) nm and yellow (570–575) bands were observed for these persistent or prolonged white light emissions. The optimal intensity Y/B ratios are likewise dependent on the crystal field in which the Dy3+ ions are located. For these samples we notice that the Y/B values varies with concentrations due to Dy3+ having different valences compared to the cations (Sr2+, Ba2+, Li+). The calculated CCT values were found to range from 5592 to 25,571 K, which makes these silicate materials ideal for outdoor activities, especially when we see cool bluish white colour emissions. Whilst the role of Dy3+ ions is for creating traps in host materials for prolonged afterglow effects, no material has ever been commercialized because of its lack of consistency in its afterglow effects and due to the degradation of the light with time [28]. These samples comprise of a mixture of electric dipole and magnetic dipole transitions as can be seen in the table. CIE was determined from both PL and TL measurements in some cases. The CIE differences between these two measurements are relatively small. The CCT values for these silicate materials fall in the cool bluish white color region. Most of these materials produce prolonged white colour emissions, with the highest being more than 1 h for the Dy3+ doped SrSiO3 silicate phosphor material, not displayed in the table.
For the SrSiO3 phosphor material, its long white light phosphorescence is due to the low trap depth that was created at 0.59 eV in the band gap of the host [4, 31]. Another silicate material that also showed a long phosphorescence white luminescence was the Sr2MgSi2O7 phosphor material with a decay time of 40 min after the UV excitation source was removed [4, 28]. Another silicate, also not mentioned in the table but in the review of Shrivastava et al. [4, 32], shows that another silicate Ca2MgSi2O7 doped with Dy3+ and with similar emission characteristics as those in Table 1 (d), but with an excitation wavelength of 254 nm, revealed a huge afterglow of 3 h and is the most preferred single doped silicate material. The decay time for all these single Dy3+ doped silicate materials show afterglow times ranging from milliseconds to a huge 3 h.
Phosphorescence White Light Emissions from Singly Doped Dy3+ Ions in Aluminate Host Materials
Dy3+ doped aluminate phosphor materials are shown in Table 2 (b). Several research papers [33,34,35,36,37] have been studied in respect of Dy3+ doped aluminate host materials, which were mostly prepared by the solid- state reaction method. The excitation wavelength has a narrow UV range from 348 to 351 nm. Typical peak emissions of blue colour were found around the 481 to 492 nm wavelength band, as well as yellow colour emissions around the 574 to 581 nm wavelength band. Magnetic dipole transitions were observed for aluminates referenced as [33], [34] and [37], while electric dipole transitions are referenced as [35] and [36], respectively. The intensity Y/B peak ratio of these samples were also found to vary with Dy3+ concentrations (Sr2+, Ca2+, Ba2+) due to the creation of defects. The Y/B value for sample referenced as [36], with similar valency as Dy3+, showed no variation with concentration but variation was observed with temperature changes. In the case of sample referenced [37], we see a change in the magnetic dipole to electric dipole transitions for variations in Dy3+ concentrations. CIE values were not calculated for all aluminate samples, but for those that were calculated, revealed phosphorescence white colour emissions with CCT values ranging from 3784 to 5951 K. These temperatures reflect warm to cool bluish white phosphorescence light emissions for selective samples. It is of interest to note that the CaAl2O4 [34] sample revealed prolonged phosphorescence white light emissions of up to 115 min.
Co-Doped Dy3+ Silicate and Aluminate Phosphor Materials for Phosphorescence White Light Emissions
While singly doped Dy3+ ions with silicate and aluminate phosphor materials are reasonably good for phosphorescence of white light emissions, the role of co-dopants ions could play a slightly different role in the choice of the dopant ions, which may or may not produce white light. Thus, for persistent white light (or other colours), it will depend on the role of Dy3+ ions in the silicate and aluminate host materials. Finding appropriate host materials for persistent luminescence is a narrow field, thus posing challenges for modern day researchers. For a long time, the ZnS: Cu phosphor material has dominated the market in terms of its persistent luminescence emissions until the emergence of more rigorous and resilient phosphor materials such as silicates and aluminates. In 1966, bright green luminescence emissions were observed in the SrAl2O4:Eu2+ [38] phosphor material and since then research has evolved in many ways yet with no commercialization to date. Later in 1996, Matsuzawa [39] co-doped this material Dy3+ and found long lasting afterglow by UV excitation and ever since more than 100 papers were published. Then came Lin et al. [32] in 2001, who fabricated Sr2MgSi2O7:Eu3+/Dy3+ phosphor material and found yet again prolonged afterglow for a silicate material. Now 25 years later, after the discovery of SrAl2O4:Eu2+/Dy3+ the search for persistent luminescent materials is still ongoing but at a snail’s pace. These two materials, SrAl2O4(aluminate)and Sr2MgSi2O7 (silicate) have dominated the research arena for decades until a novel long lasting and persistent (consistently) luminescence material is proposed for phosphorescence emissions. Another, aluminate material in the family of alkaline earth metals, MAl2O4 (M = Ca, Sr, Ba) has received considerable attention due to its persistent phosphorescence characteristics [7]. The aluminate Sr4Al14O25:Eu2+/Dy3+ [40] phosphor material, has produced prolonged phosphorescence luminescent emissions of up to 20 h. There is no consensus from all the research thus far, whether co-doping has impacted on the afterglow effects. Further, synthesis methods have proven to be crucial in afterglow effects, with the solid-state reaction method leading the race, followed by the combustion method. The role of Dy3+ ions is merely to create traps, particularly low traps of about 0.65 eV in the host materials, that would be ideal for phosphorescence luminescence [38].
Many research papers of co-doped Eu2+/Dy3+ aluminates have been analyzed in the review paper of Eeckhout et al. in 2010 [38] for materials that show persistent luminescence long after the UV excitation source is removed. The following aluminates, with afterglow times given in brackets, shows the prominent role played by aluminates, and they are: SrAl2O4:Eu2+/Dy3+ [38, 41] (more than 30 h), BaAl2O4:Eu2+/Dy3+ [38, 42] (more than 2 h), Sr4Al14O25:Eu2+/Dy3+ [38, 43] (more than 20 h), SrAl4O7:Eu2+/Dy3+ [38, 44] (more than 3 h), SrAl12O19:Eu2+/Dy3+ [38, 45] (more than 3 h) and SrMgAl10O17 [38, 46] (more than 3 min).
What was interesting from this review paper was that when BO4 was incorporated [47] into SrAL2O4:Eu2+/Dy3+, the trap depth appears to have decreased from 0.79 eV to 0.65 eV, allowing for prolonged phosphorescence luminescence.
Further in the review paper of [38], prolonged phosphorescence properties of silicate co-doped materials are discussed. The after-glow times of these silicate materials are given in brackets: SrMgSi2O7:Eu2+/Dy3+ [38, 48] (more than 10 h), SrMgSi2O8:Eu2+/Dy3+ [38, 49] (more than 10 h), Ca3MgSi2O8:Eu2+/Dy3+ [38, 50] (more than 5 h), Ba3MgSi2O8:Eu2+/Dy3+ [38, 50] (more than 1 h), CaMgSi2O6:Eu2+/Dy3+ [38, 51] (more than 4 h) and Sr2Al2SiO7:Eu2+/Dy3+ [38, 52] (more than 2 h).
Mechanism of Energy Transfer of Dy3+ Codoped Phosphor Host Materials
Many models have been postulated since 1996 to explain the mechanism of persistent luminescence in many of the above host materials but none have a uniform strategy on the precise mechanism of energy transfer. Besides the accepted theoretical model of charges being excited and getting caught in “trap states” within the band gap of host materials, and after thermal stimulation, they get “detrapped” leading to the afterglow effects observed for this selective class of materials. About two decades of research has been done on the first SrAl2O4:Eu2+, Dy3+ phosphor material for prolonged afterglow effects (more than 30 h). The first ever model on energy transfer was proposed by Matsuzawa et al. [39] in 1996. They proposed that holes be the prominent charge carrier. For the selective SrAl2O4:Eu2+, Dy3+ phosphor material, they indicated that when Eu2+ is excited, holes escape to the valence band, leaving behind a monovalent Eu+ charge, which is captured by the Dy3+ ion within the band gap of the host, creating a tetravalent Dy4+ ion charge. After thermal stimulation (kT), the trapped hole then migrates back to the valence band and back to the monovalent Eu+ ion, this process can be regarded as return to the ground state of the divalent Eu2+ ion, through emission of a photon that accounts for the afterglow effects. This process could similarly be explained with direct transition within the band gap through recombination of electron-hole effects [34]. Later in 2003, Aitasalo et al.. [53] used a non Dy3+ doped CaAl2O4:Eu2+, Nd3+ sample to explain persistent luminescence, they proposed that electrons escape directly from the valence band of the host to various unknown traps within the band gap region, a hole is created from this process in the valence band. This hole then moves within the valence band where it is captured by a calcium vacancy in the band gap region. By thermal stimulation (kT), the electrons from various trap levels then migrate to an oxygen vacancy, then by recombination with the hole calcium site, energy is released and whose energy is then used to excite Eu2+ from its ground state to the 5d1 state of Eu2+ ion, which is then de-excited to its ground with an emission of a photon [34, 53]. Later in 2005, Doren et al. [54] proposed their method for persistent luminescence, using the SrAl2O4:Eu2+, Dy3+ phosphor material but refuted claims made by Aitasalo et al. They stated that electrons are excited from the ground state of Eu2+ ion to its upper excited 5d1 state, and because they are so close to the conduction band, which then allows for easy migration into the conduction band, after which they are trapped by a trivalent ion such as Dy3+ ion, creating a divalent ion. Thermal excitation releases this trapped electron which then moves to the conduction band and then recombines with the luminescent 5d1 state and after de-excitation moves to the ground state of Eu2+ with an emission of a photon [34]. Later, at the same time, another model was proposed by Clabau et al. [55] on the same material as above, appears to be very simplistic in nature. Firstly, an electron is excited from the ground state of Eu2+ ion to its upper excited state 5d1. This electron then migrates to an oxygen vacancy (defect site) and after thermal stimulation, the electron then moves back to its luminescent center at 5d1 and after de-excitation moves back to its ground state with an emission of a photon (without moving along the conduction band, as in the case of Doren et al’s model).
Some models proposed currently are from two research groups, one by Castaing et al. [56] and another by Tanaka et al. [57], both published in 2021, shows departures from the earlier models proposed a decade ago on the same SrAl2O4:Eu2+, Dy3+ phosphor material. In the case of the Tanaka et al. model, electrons are first excited from the ground state of the Eu2+ ion to excited energy levels just below the conduction band, and through migration into the conduction band, they are then captured by “trap” levels below the conduction band. By thermal stimulation, they then make another migration (detrapping) through the conduction band back to the luminescent center and then are de-excited to the ground state of Eu2+ ion (recombination of electron-hole) though the release of a photon of energy. This model is very simplistic in nature as well. The work of Castaing et al., is a simple process whereby charges are excited to the 5d1 energy level of Eu2+ ion from its ground state, which lies just below the conduction band of the host material. This electron is then trapped at the storage site (charge trapping) and by thermal excitation, it is released back to the luminescent center (5d1) and is then de-excited to its ground state through the release of a photon of energy.
Conclusion
For Dy3+ ions, two separate transitions; namely, a blue 4F9/2 →6H15/2 and a yellow 4F9/2→ 6H13/2 are responsible for white colour emissions, if the Y/B intensity ratios are carefully optimized. However, the role of Dy3+ ions is not only for white light emissions for LED applications, but it can also play a fundamental role in persistent luminescence when co-doped with dopants pairs such as Eu2+, Eu3+, Ce3+ ions. Due to the lack of the red colour ingredient of white light, red emitting dopants such as Sm3+ or Eu3+ have played a huge role in ameliorating the situation. Many factors must be taken into consideration in producing enhanced white luminescence for prolonged colour emissions. Factors such as cost effectiveness, chemical and thermal stability, appropriate choice of host compounds and choice of dopants should be on the top of the list in this regard. In respect of these factors, the position of the Dy3+ ions in the host material, either at symmetric or asymmetric locations in the host compound, will determine the nature of the transitions as well as its CIE and CCT values. Several host materials have been considered in this respect and they range from phosphates to silicates and aluminates mainly because of their excellent chemical and stability characteristics. In respect of singly doped Dy3+ ions in phosphate host materials, the dominant blue magnetic dipole transitions in different host materials produces white light whose CCT values are generally more than 6000 K, making it ideal for energetic outdoor settings. On the other hand, white colour emissions due to electric dipole transitions (yellow colour dominant), their CCT values are in the range from 3000 to 6800 K, making it ideal for both indoor (warm) and outdoor (cool bluish white) settings. The situation is quite different when Dy3+ ions are co-doped with different phosphate materials. When co-doped with Eu3+ ions in the phosphate host, it appears that their CIE and CCT values are tuned with concentration changes, which mainly results from the energy transfer from Dy3+ ions to Eu3+ ions. An analogous situation was observed when Dy3+ ions was co-doped with Sm3+ ions. However, something strange happens when Dy3+ ions is co-doped with Ce3+ ions. In this scenario, we observe a change in the dipole transition from magnetic to electric dipole transition when comparisons are made with singly doped Ca3Mg(PO4)4:Dy3+ to co-doped Ca3Mg(PO4)4:Ce3+/Dy3+ ions, resulting in the energy transfer from Ce3+ ions to Dy3+ ions. The CCT values for white colour emission drops when the magnetic dipole transition moves over to the electric dipole transition from this co-doping effect. The search for persistent luminescent materials has led us to exclusively to study two types of host materials; namely, silicates and aluminates, which has been extensively studied in literature. The role of the Dy3+ ions in this scenario is in the creation of energy traps within the band gap of the host material. Since the discovery of SrAl2O4:Eu2+/Dy3+ and Sr2MgSi2O7:Eu2+/Dy3+ phosphor materials for prolonged luminescence, the search for better materials is just around the corner. Singly doped Dy3+ silicate materials appear to emit white light ranging from milliseconds to more than an hour for SrSiO3 and to a high 3 h for the Ca2MgSi2O12 phosphor material. Whilst these transitions are comprised of both magnetic and electric dipole transitions, their CCT values are above 5500 K and indicative of prolonged luminescence for outdoor settings. Whilst not much pertinent information is given about single doped aluminates, they appear to have their CCT values to be less than 6000 K and more than adequate for cool to warm white lighting for indoor settings. As far as phosphorescence white light emissions in aluminates are concerned, only CaAl2O4 has revealed prolonged luminescence of more than 115 min. On the other hand, aluminates and silicates are co-doped with other rare –earth ions (such as Eu2+), we notice enhanced phosphorescence of up to 30 h for the SrAl2O4:Eu2+/Dy3+ phosphor material and 20 h for another aluminate Sr4Al14O25:Eu2+/Dy3+ phosphor material, with others having a decay time of at least an hour. This points to the fact the intricate role of Eu2+ when doped with Dy3+ ions in producing persistent phosphorescence.
Availability of data material
N/A.
References
Nair GB, Dhoble SJ (2020) The Fundamentals and applications of light-emitting diodes. Woodhead Publishing. Chap. 4
Cho J, Park JH, Kim JK, Schubert EF (2017) White light-emitting diodes: history, progress, and futures. J Photonic Review 11(2):1600147
Zho Y, Xu H, Zhang X, Zhu G, Yan D, Yu A (2015) Facile synthesis of YAG: Ce3+ thick film for phosphor converted white light emitting diodes. J Eur Ceram Soc 35:3761–3764
Shrivastava R, Kaur J, Dubey V (2016) White Light Emissions by Dy3+ Doped Phosphor Matrices: a short review. J Fluoresc 26:105–111
Su Q, Pei Z, Chi L, Zhang H, Zhang Z, Zou F (1993) The yellow-to-blue intensity ratio (Y/B) of Dy3+ emission. 192:25–27
Dutczak DA Eu2 + activated persistent luminescent materials, PhD Thesis, Debye Institute, University of Utrecht, Netherlands
Fernandez-Rodriguez L, Duran A, Pascual MJ (2021) Silicate-based persistent phosphors 7:100150
Shasmal N, Karmakar B (2019) White light-emitting Dy3+ - doped transparent chloroborosillicate glass: synthesis and optical properties. J Asian Ceam Soc 7(1):42–52
Wang J, Wang J, Duan P (2013) Luminescent properties of Dy3+ doped Sr3Y(PO4)3 for white LEDs. 107:96–98
Ratnam BV, Jayasimhadri M, Jang K, Lee HS (2010) White Light Emission from NaCaPO4: Dy3+ phosphor for Ultraviolet-Based White Light Emitting Diodes. J Am Ceram Soc 93:38–3861
Zhang ZW, Liu L, Zhang XF, Ping J, Zhang, Zhang WG, Wang DJ (2015) Preparation, and investigation of CaZr4(PO4)6: Dy3+ single-phase full-color phosphor. Specrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 137:1–6
Hu Z, Meng T, Zhang W, Ye D, Cui Y, Luo L, Wang L (2014) Synthesis, and luminescence of Dy3+-activated NaSrPO4 phosphors for novel white light generation. 25:1933–1937
Xu Q, Sun J, Cui D, Di Q, Zeng J (2015) Synthesis and luminescence properties of novel Sr3Gd(PO4)3: Dy3+ phosphor. J Lumin 158:301–305
Zhang ZW, Song AJ, Yue Y, Zhong H, Zhang XY, Ma MZ, Liu RP (2015) White light emission from Ca9Bi(PO4)7:Dy3+ single-phase phosphors for light-emitting diodes. 650:410–414
Liu F, Liu Q, Fang Y, Zhang N, Yang B, Zhao G (2015) White light emission from NaLa(PO3)4:Dy3+ single-phase phosphors for light-emitting diodes. Ceram Int 41:1917–1920
Liu Q, Liu Y, Ding Y, Peng Z, Tian X, Dong QYG (2014) A white light emitting luminescent material Ba3Y(PO4)3: Dy3+. Ceram Int 40:10125–10129
Chemingui S, Ferhi M, Naifer KH, Ferid M (2015) Synthesis and luminescence characteristics of Dy3+ doped Kla(PO3)4. J Lumin 166:82–87
Zhu G, Li ZW, Wang C, Zhou FG, Wen Y, Xin SY (2017) Electronic structure and photoluminescence property of a novel white emission phosphor Na3MgZr(PO4)3:Dy3+ for warm white light emitting diodes. Chin Phys B 9:097801
Zhu G, Wang Y, Wang Q, Ding X, Geng W, Shi Y (2014) A novel white emitting phosphor of Dy3+ doped Ca19Mg2(PO4)14 for white light-emitting diodes. J Lumin 154:246–250
Reddy AA, Sekhar MC, Pradeesh K, Babu SS, Prakesh GV (2011) Optical properties of Dy3+ - doped sodium-aluminium-phosphate glass. J Mater Sci 46:2018–2023
Nagpure IM, Saha S, Dhoble SJ (2009) Photoluminescence and thermoluminescence characterization of Eu3+ - and Dy3+ -activated Ca3(PO4)2 phosphor. J Lumin 129:898–905
Yan W, Li J, Zhang W, Gao X, Zhang P (2021) Warm-white luminescence of Dy3+ and Sm3+ co-doped NaSrPO4 phosphors through energy transfer between rare earth ions. J Mater Sci 32:16648–16661
Xu M, Wang L, Jia D, Liu L (2015) Enhancing the luminescent properties of Zn2P2O7:Ce3+, Dy3+ via efficient energy transfer. Mater Res Bull 70:691–696
Nair GB, Dhoble SJ (2017) White light emission through efficient energy transfer from Ce3+ to Dy3+ ions in Ca3Mg3(PO4)4 matrix aided by Li+ charge compensator. J Lumin 192:1157–1166
Rodriguez LF, Balda R, Fernandez J, Duran A, Pascual MJ (2022) Role of Eu2+ and Dy3+ Concentration in the Persistent Luminescence of Sr2MgSi2O7 Glass-Ceramics, Materials, 15 3068
Sahu IP, Chandrakar P, Baghel RN, Bisen DP, Brahme N, Tamrakas RK (2015) J Alloys Compd 649:1329–1338
Bisen DP, Badapanda T, Choubey A, Patle Y, Chandrawanshi E (2021) Synthesis and optical characterization of Dy3+ doped barium alumino silicate phosphor. Mater Sci Eng B273:115445
Liu B, Kong L, Shi C (2007) White-light long-lasting phosphor Sr2MgSi2O7:Dy3+,Journal of luminescence, 122 – 13121–124
Sunitha DV, Nagabhushana H, Sharma SC, Nagabhushana BM, Daruka D, Prasad, Chakradhar RPS (2014) Study on low temperature solution combustion synthesized Sr2SiO4:Dy3+ nano phosphor for white LED. Spectrochim Acta Part A Mol Biomol Spectrosc 127:381–387
You P, Yin G, Chen X, Yue B, Huang Z, Liano X, Yao Y (2011) Luminescence properties of Dy3+ doped Li2SrSiO4 for NUV- excited LEDs. Opt Mater 33:1808–1812
Kuang J, Liu Y, Zhang J (2006) White-light-emitting long lasting phosphorescence in Dy3+ -doped SrSiO3. J Solid-State Chem 179:266–269
Lin L, Weiping Z, Zhiquiang Z, Min Y (2009) Photolumiescence properties and thermoluminescence curve analysis of a new white long-lasting phosphor: Ca2MgSi2O7:Dy3+. J Rare Earths 27:749–752
Pavani K, Kumar JS, Sasikala T, Jamalaiah BC, Seo HJ, Moothy LR (2011) Luminescent characteristics of Dy3+ doped strontium magnesium aluminate. Mater Chem Phys 129:272–295
Zhai BG, Chen MM, Huang YM (2022) Photoluminescence, and afterglow of Dy3+ doped CaAl2O4 derived via sol-gel combustion. RSC Adv 12:31757–31768
Deng B, Chen J, Zhu CS, Liu H (2019) A novel UV pumped yellow-emitting phosphor Ba2YAlO5:Dy3+ for white-emitting diodes, E3S Web of Conferences, 79 03005
Ji Y, Cao J, Zhu Z, Wang Y, Tu C (2012) Synthesis, and white light emission of Dy3+ ions doped hexagonal structure YAlO3 nanocrystalline. J Lumin 132:702–706
Yang F, Ma H, Liu Y, Han B, Yu Q (2014) Photoluminescence properties of novel Dy3+ doped Ba5CaAl4O12 phosphors. Ceram Int 40:10189–10192
Eeckhout KVD, Smet PF, Poelman D (2010) Persistent luminescence in Eu2+ - Doped Compounds. Rev Mater 3:2536–2566
Matsuzawa T, Aoki Y, Murayama N (1996) A new long phosphorescent phosphor with high brightness, SrAl2O4: Eu2+, Dy3+. J Electrochem Soc 118:2670–2673
Zhao C, Chen D, Yuan Y, Wu M (2006) Synthesis of Sr4Al14O25:Eu2+, Dy3+ phosphor nanometer powders by Combustion processes and its Optical Properties. Mater Sci Engineering: B 133:200–204
Katsumata T, Nabae T, Sasajima K, Komuro S, Morikawa S (1997) Effects of composition on the long phosphorescent SrAl2O4: Eu2+, Dy3+ phosphor crystals. J Electrochem Soc 144:L243–L245
Sakai R, Katsumata T, Komuro S, Morikawa T (1999) Effects of composition on the phosphorescence from BaAl2O4:Eu2+, Dy3+ crystals. J Lumin 85:149–154
Lin Y, Tang Z, Zhang Z (2001) Preparation of long-afterglow Sr4Al14O25 – based luminescent material and its optical properties. Mater Lett 51:14–18
Preethi KRS, Lu CH, Thirumalai J, Jagannathan R, Natarajan TS, Nayak NU, Radhakrishna I, Jayachandran M, Trivedi DC (2004) SrAl4O7:Eu2+ nanocrystals: synthesis and fluorescence properties. J Phys D-Appl Phys 37:2664–2669
Katsumata T, Sasajima K, Nabae T, Morikawa S (1998) Characteristics of strontium aluminate crystals used for long-duration phosphors. J Am Ceramic Soc 81:413–416
Wanjun T, Donghua C, Ming W (2009) Luminescence studies on SrMgAl10O17:Eu, Dy phosphor crystals. Opt Laser Technol 41:81–84
Nag A, Kutty TRN (2003) Role of B2O3 on the phase stability and long phosphorescence of SrAl2O4:Eu. J Alloys Compd 354:221–231
Lin Y, Tang Z, Zhang Z, Wang X, Zhang J (2001) Preparation of a new long afterglow blue-emitting Sr2MgSi2O7- based photoluminescent phosphor. J Mater Sci Lett 20:1505–1506
Lin Y, Tang Z, Zhang Z, Nan CW (2003) Luminescence of Eu2+ and Dy3+ activated R3MgSi2O8- based (R = ca, Sr, Ba) phosphors. J Alloys Compd 348:76–79
Lin Y, Zhang Z, Tang Z, Wang X, Zhang J, Zheng Z (2001) Luminescent properties of a long new afterglow Eu2+ and Dy3+ activated Ca3MgSi2O8 phosphor. J Eur Ceramic Soc 21:683–685
Jiang L, Chang C, Mao D (2003) Luminescent properties of CaMgSi2O6 and Ca2MgSi2O7 phosphors activated by Eu2+, Dy3+ and Nd3+. J Alloys Compd 360:193–197
Ding Y, Zhang Y, Wang Z, Li W, Mao D, Han H, Chang C (2009) Photoluminescence of Eu single doped and Eu/Dy codoped Sr2Al2SiO7 phosphors with long persistence. J Lumin 129:294–299
Aitasalo T, Deren P, Holsa J, Jungner H, Krupa JC, Lastusaari M, Legendziewicz J, Niittykoski J, Strek W (2003) Persistent luminescence phenomena in materials doped with rare earth ions. Solid State Chem 171:114–122
Dorenbos P (2000) Mechanism of persistent luminescence in Eu2+ and Dy3+ codoped aluminate and silicate compounds. J Electrochem Soc 152:H107–H110
Clabau P, Rocquefelte X, Jobic S, Deniard P, Whangbo MH, Garcia A, Le Mercier T (2005) Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+- doped SrAl2O4 with codopants Dy3+ and Bi3+. Chem Mat 17:3904–3920
Castaing V, Arroyo E, Becerro A, Ocana M, Lozano G, Miguez H (2021) Persistent luminescent nanoparticles: Challenges and opportunities for a shimmering future. J Appl Phys 130:080902
Tanaka R, Uematsu K, Sato M, Toda K (2021) Afterglow improvement of high concentration Dy3+ co-doped SrAl2O4:Eu2+ phosphor prepared by H3BO3 free synthesis using melt quenching method. 129(7):372–376
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Contributions
I prepared this manuscript myself.
Corresponding author
Ethics declarations
Ethics approval
N/A.
Competing interests
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reddy, L. A Review of the Efficiency of White Light (or Other) Emissions in Singly and Co-Doped Dy3+ Ions in Different Host (Phosphate, Silicate, Aluminate) Materials. J Fluoresc 33, 2181–2192 (2023). https://doi.org/10.1007/s10895-023-03250-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03250-y