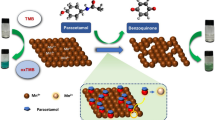
Abstract
Highly fluorescent nitrogen doped carbon quantum dots (N-CQDs) were prepared by a single-step method based on microwave heating of cane sugar and urea. The produced N-CQDs were applied as nano-sensors for the spectrofluorimetric determination of eplerenone and spironolactone. A strong emission band at 376 nm was obtained after excitation at 216 nm due to the produced N-CQDs. The native fluorescence of N-CQDs was obviously quenched upon adding increased concentrations of each drug. A strong correlation was found between the fluorescence quenching of N-CQDs and the concentration of each drug. The method was found to be linear over the range of 0.5 to 5.0 μg/mL for eplerenone and 0.5 to 6.0 μg/mL for spironolactone with LOQ of 0.383 μg/mL and 0.262 μg/mL. The developed method was further extended for determination of both drugs in their pharmaceutical tablets and spiked human plasma. The results obtained were statistically compared with those of reported methods. The mechanism of fluorescence quenching of N-CQDs by the two drugs was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eplerenone (EPL) and spironolactone (SPR) belong to the class of potassium-sparing diuretics. Both drugs are prescribed for hypertension and heart failure. Eplerenone chemically is: 9,11α-Epoxy-7α-(methoxycarbonyl)-3-oxo-17α-pregn-4-ene-21,17-carbolactone [1] (Supplementary materials Fig. S1). It is an aldosterone receptor antagonist which helps in reducing the risk of myocardial infarctions and lowering blood pressure [2]. Spironolactone chemically is: (2`R)-7α-(Acetylsulfanyl)-3`,4`-dihydro-5`H-spiro[androst-4-ene-17,2`-furan]-3,5`-dione (Supplementary materials Fig. S2) [1]. It is necessary to observe their plasma levels mainly in patients who suffer from kidney problems as both drugs can cause serious complications. Many analytical methods for EPL estimation were reported, including spectrophotometry [3,4,5], LC–MS [6], RP-HPLC [7,8,9], HPTLC [10] and spectrofluorimetry [11]. Many analytical techniques have been used for the estimation of SPR, either in pharmaceutical preparations or biological fluids included; spectrophotometry [12,13,14], HPLC [15,16,17], HPTLC [18] and spectrofluorimetry [19]. Most of these analytical methods are multi-steps procedures and require expensive instruments (Table 1). Nowadays, nano-sensors and their pharmaceutical applications gain the greatest interest. For many years, semiconductor quantum dots have been extensively investigated for their strong and tunable fluorescence emission properties, which enable their applications in biosensing and bioimaging. However, semiconductor quantum dots possess certain limitations such as high toxicity due to the use of heavy metals in their production. It is known that heavy metals are highly toxic even at relatively low levels, which may prove prohibitive to any clinical studies. This prompted the creation of CQDs to replace semiconductor quantum dots [20]. Carbon quantum dots (CQDs) are classified as fluorescent nano particles which are characterized by their low cost, photostability, biocompatibility, high water solubility, safety and being nontoxic [21,22,23,24]. Moreover, doping of heteroatoms as nitrogen atom to the structure of CQDs enhances the optical and electrical properties of CQDs [25, 26].
The current work aimed to develop a sensitive spectrofluorimetric method for determination of EPL and SPR in commercial tablets and spiked human plasma with no need for any derivatization. The method of preparation of N-CQDs depends on microwave heating of aqueous solution of cane sugar as carbon source and urea as nitrogen source. Both drugs showed quantitative quenching of the fluorescence of N-CQDs.
The main advantage of the proposed method is the ability of the method to produce N-CQDs with quantum yield of 0.57 in less than thirty minutes and its ability to detect very low concentrations of the studied drugs.
Experimental
Reagents and Materials
-
Methanol (HPLC grade) was obtained from Fisher in Belgium.
-
Glacial acetic acid (99%) was obtained from Alfa chemical in Egypt.
-
Boric acid and orthophosphoric acid (85%) were obtained from Sigma-Aldrich in Switzerland.
-
Britton Robinson Buffer was made up of mixture of 0.04 M boric acid, 0.04 M acetic acid and 0.04 M phosphoric acid, then 0.2 M sodium hydroxide was used to adjust pH range from 2.1 to 12.
-
Deionized water was used during the course of the work.
-
Spironolactone (purity of 99.9%) was obtained from Kahira Pharm. &Chem. Company Cairo in Egypt.
-
Eplerenone (purity of 99.9%) was kindly provided by NODCAR, Cairo, Egypt.
-
From local pharmacies; Tensopleron 25 mg tablets (B.N.125304) and Tensopleron 50 mg tablets (B.N.125402), products of Global Napi Pharmaceuticals, Spectone® 25(Batch N0.2110863) and Spectone® 100 mg (Batch N0.2110871) products of Kahira Pharm. & Chem. Company, Cairo, Egypt.
-
Human plasma samples were provided by Mansoura University Hospital, Mansoura, Egypt. The samples kept frozen until use at − 80 °C.
Apparatus
-
Shimadzu RF-6000 series Spectrofluorometer equipped with Xenon lamp (150 W) has been used for fluorescence measurements in the wavelength range from 200 to 600 nm. The apparatus was operated at high sensitivity mode during the entire work.
-
JEM-2100 high-resolution transmission electron microscope (HRTEM) (JEOL, Tokyo) operated at 200 kV to investigate the morphology of N-CQDs.
-
FT-IR Spectrometer (Thermo Scientific Nicolet—iS10) (MA, USA).
-
Shimadzu UV-1900i Spectrophotometer (Japan) has been used for spectrophotometric measurements in the wavelength range from 200 to 600 nm.
-
Elmasonic S100 (H) Ultrasonic bath (Germany).
-
ZX3 vortex mixer (Velp. Scientific) (Italy).
-
Jenway 3510 pH-Meter (England).
-
Centrifuge, model 2-16P (Germany).
-
Domestic LG microwave (Frequency of 2,450 MHz and output of 900 W) (Model No: MH7043BARS) has been used for synthesis of N-CQDs.
Preparing Solutions
Preparing stock solutions (1000.0 µg/mL) of EPL and SPR was carried out by dissolving 10.0 mg of each drug separately in 10.0 mL of methanol. Stock solutions were diluted by one-tenth to prepare working solutions (100.0 µg/mL). The working solutions were then used to prepare different concentrations.
Synthesis of fluorescent N-CQDs
A domestic microwave was used to prepare the N-CQDs by heating fifteen grams of cane sugar and three grams of urea dissolved in 30.0 mL deionized water. The solution was heated for ten minutes till complete charring. Then, the formed N-CQDs were left at room temperature to cool down. Water was used to dilute the produced N-CQDs. Centrifugation was carried out at 6000 rpm for ten minutes to remove suspended particles. Then, the impurities were removed by 0.22 µm Syringe filters. The N-CQDs were transferred to 100.0 mL volumetric flask and the volume was completed to the mark with deionized water to prepare stock solution.
Quantum Yield
The single point method was used to calculate the quantum yield of N-CQDs according to Eq. 1 [27]:
In which:
Φ represents the quantum yield, F, A and η stand for the integrated measured intensity of emission, the absorbance and the solvent refractive index. The subscripts x refers to the unknown sample and st refers to the reference samples. In aqueous solutions, ηx/ηst is equal to 1. Quinine sulfate was used as the standard and dissolved in 0.1 M H2SO4 (QY: 0.54). The produced N-CQDs exhibit high quantum yield (0.57).
General Procedures
Raw Materials
In this study, different aliquots from working standard solutions (100.0 μg/mL) of both drugs were transferred to a series of 10.0 mL volumetric flasks containing 400 μL aliquots of N-CQDs. 1 mL of BRB of pH 3.0 for EPL and 1 mL of BRB of pH 5.0 for SPR were added to each flask. Deionized water was used to complete each solution to the mark. To create the calibration curves, the quenching values of fluorescence (ΔF) at 376 nm were plotted against final drug concentrations in (μg/mL). As an alternative, the regression equations were derived.
Commercial Tablets
Tensopleron Tablets
Ten Tensopleron tablets were weighed and pulverized. An amount equivalent to 10.0 mg of EPL was transferred into a 10.0 mL volumetric flask then five milliliters methanol were added. Sonication was performed for thirty minutes. The content of the flask was completed to the volume with methanol and filtered to give a solution of 1000.0 μg/mL. Then 1.0 mL was transferred from the stock solution to 10.0 mL volumetric flask and diluted with methanol to give solution of 100.0 μg/mL. The procedure described above (“Raw Materials” section) was applied. Percentage recoveries were determined using the corresponding regression equation.
Spectone® Tablets
Ten Spectone® tablets were weighed and pulverized. An amount equivalent to 10.0 mg of SPR was accurately transferred into 10.0 mL volumetric flask then five milliliters of methanol were added. Sonication was performed for thirty minutes. The content of the flask was completed to the volume with methanol and filtered to give a solution of 1000.0 μg/mL. Then 1.0 mL was transferred from the stock solution to 10.0 mL volumetric flask and diluted with methanol to give solution of 100.0 μg/mL. The procedure was completed as described under “Raw Materials” section. Percentage recoveries were determined using the corresponding regression equation.
Spiked Human Plasma
Aliquots of EPL and SPR standard working solutions were accurately added to 1.0 mL human plasma in a series of 15.0 mL centrifuge tubes. After vortexing each tube for thirty seconds, the volume was made up to 5.0 mL with acetonitrile. Then centrifugation was performed at 3600 rpm for thirty minutes. Different volumes of the supernatant were transferred into 10.0 mL volumetric flasks and the described procedure (“Raw Materials” section) was followed. The percentage recoveries were calculated adopting the corresponding regression equation.
Results and Discussion
The recent study presents rapid, simple, economic and sensitive spectrofluorimetric method for quantitative estimation of both EPL and SPR. The method of synthesis of N-CQDs is ecofriendly, rapid, facile and need simple procedure and available precursors. The method of preparation needs thirty minutes to yield hydrophilic and highly fluorescent probe which could be directly used in estimation of EPL and SPR.
Characterization of N-CQDs
Microwave assisted method was used for synthesis of water-soluble N-CQDs using cane sugar and urea as carbon and nitrogen sources. A solution of orange color was produced indicating the formation of N-CQDs. The general procedure for synthesis of N-CQDs is illustrated in (Scheme 1). The N-CQDs showed spherical particles with diameters in the range of 5.5 nm to 9.8 nm with an average diameter of 7.7 nm as presented in TEM image (Fig. 1). FT-IR analysis was also used to identify functional groups of N-CQDs (Fig. 2). The broad band in the range of 3600– 3100 cm−1, the peaks at 2064, 1638 cm−1 and the vibrations at 1265 cm−1 represent N–H and O–H groups, C = C/C = O, C-N and the stretching modes of C–O–C band, respectively [28]. Also, the peak at 695 cm−1 represents C-H. The absorption spectrum of N-CQDs is shown in (Supplementary material Fig. S3). The excitation and emission spectra of the fluorescent N-CQDs are presented in (Supplementary Material Figs. S4 and S5), high fluorescence intensity of N-CQDs at λem = 376 nm (λex = 216 nm) and the quenching effect of each of EPL and SPR on N-CQDs are obvious as shown in (Fig. 3a and b). The fluorescence quenching of N-CQDs by EPL and SPR is illustrated in (Scheme 2).
a Fluorescence emission spectra of the N-CQDs in aqueous solution upon addition of various concentrations of EPL (from top to bottom: 0 μg/mL, 0.5 μg/mL, 1.0 μg/mL, 2.0 μg/mL, 3.0 μg/mL, 4.0 μg/mL, 5.0 μg/mL), b. SPR (from top to bottom: 0 μg/mL, 0.5 μg/mL, 1.0 μg/mL, 2.0 μg/mL, 3.0 μg/mL, 5.0 μg/mL, 6.0 μg/mL)
Mechanism of fluorescence response of N-CQDs to both EPL and SPR
Stern–Volmer’s equation was applied to analyze the fluorescence emission intensity of the N-CQDs-EPL and N-CQDs-SPR systems for a better understanding of the fluorescence quenching mechanism [29].
In this equation, F and F0 refer to the fluorescence intensities in the presence and absence of quencher, respectively. Kq and Ksv stand for the quenching rate and the Stern–Volmer quenching constants, τ0 is the average fluorescence lifetime (10− 8 s) and [Q] is the concentration of the quencher.
The quenching experiments were performed at 298 K. The quenching results were applied to the previous equation resulted in Kq values of 5.7 × 1011 for EPL and 1.06 × 1012 for SPR. The two values were larger than (2.0 × 1010 L.mol−1.s−1) which is the maximum diffusion rate constant [30]. As a result, the quenching mechanism is assumed to be static.
In this study, an overlap exists between the excitation spectrum of NCQDs and UV–VIS absorption spectrum of EPL and SPR (Supplementary material Fig. S6, S3). Therefore, Inner Filter Effect (IFE) might occur. The correction of N-CQDs fluorescence intensity for potential IFE was studied. The following equation was used to calculate the corrected fluorescence intensity Eq. (3) [31]:
In which:
Fobs represents the observed fluorescence intensity, Fcorr is the corrected fluorescence intensity after removing inner filter effect from Fobs, Aem and Aex are the absorbance of the drug at the emission and excitation wavelengths of N-CQDs. Equation 4 was used to calculate the suppressed efficiency (%E) for observed and corrected fluorescence intensities.
The plot between %E against drug concentration showed that IFE affect by 15.3% and 9.36% in the quenching of N-CQDs fluorescence intensity by EPL and SPR (Fig. 4).
Performance Optimization
Different factors that affect the fluorescence sensing of EPL and SPR have been investigated to choose the optimum conditions.
Effect of pH and Volume of the Buffer
The influence of different volumes of BRB solution on fluorescence intensity quenching by EPL and SPR over pH range of 2.1–12 was studied. The highest ΔF value was achieved using 1 mL of the buffer of pH 3 for EPL and 1 mL of BRB of pH 5 for SPR (Supplementary material Fig. S7).
Effect of volume of N-CQDs
The maximum quenching of fluorescence intensity was attained by using a volume of 0.4 mL of N-CQDs with both drugs (Supplementary material Fig. S8).
Effect of Temperature and Incubation Time
The experiment was tried at different temperatures (298, 303 and 313 K) and the fluorescence intensities were recorded at the emission wavelength. It was noticed that there was no significant difference in the relative fluorescence intensities, so the experiment was carried out at 298 K (Supplementary material Fig. S9). Also, fluorescence spectra after adding the studied drugs to N-CQDs were recorded at different time intervals starting from one to forty minutes. It was found that the reaction between N-CQDs and the studied drugs was instantaneous and completed in nearly one minute. The fluorescence values were constant for thirty minutes.
Method Validation
ICHQ2(R1) Guidelines were followed to prove that the method is valid [32].
The range and linearity of the suggested method were evaluated by plotting relative quenched fluorescence intensities versus concentrations of each drug in μg/mL. The method was found to be linear over the range of 0.5 to 5.0 μg/mL for EPL and 0.5 to 6.0 μg/mL for SPR (Supplementary material Fig. S10). The following equations represent the linear regression, Eqs. (5) and (6):
where C is the concentration of each drug in μg/mL and F0, F are the fluorescence intensity of N-CQDs in the absence and presence of both drugs, respectively.
The proposed method is adequately sensitive for quantitative estimation of the analytes in biological fluids due to the relatively small values of LOD and LOQ (Table 2).
The calculated mean % recoveries of raw materials of EPL and SPR within the specified concentration ranges for the comparison and proposed method guaranteed the accuracy of the method. The comparison method for EPL [5] depends on absorbance measurement at 241 nm in (30% v/v) methanol. While the comparative method for SPR [12] is based on measuring its absorbance in methanol at 237 nm. Variance F-test and Student’s t-test were applied to evaluate the results attained from the proposed method and the comparative method as presented in (Supplementary material Table S1) [33]. There was no significant difference between the methods in terms of precision and accuracy.
The intra-day and inter-day precisions were investigated to ensure the precision of the method. The values of %RSD were calculated using three different concentrations and each concentration was replicated three times. Both drugs showed values of % RSD less than 2.0% (Table 3).
The robustness of the method was evaluated by making minor deliberate changes in conditions of the experiment. The volume of buffer was varied by ± 0.2 mL and the pH by ± 0.2. There was no difference with those small variations which may take place throughout regular work. % Recoveries of the studied drugs in their commercial tablets were estimated to confirm the selectivity of the suggested method as presented in (Supplementary material Tables S2, S3). The results showed that common tablet excipients did not interfere with the analysis of both drugs, so the method is valuable for the estimation of EPL and SPR in their commercial tablets.
Applications
Assay of EPL and SPR in their Pharmaceutical Tablets
The proposed method was successfully employed to estimate EPL and SPR in their commercial tablets. The results in (Supplementary material Tables S2 & S3) show excellent agreement with those obtained from the comparison methods [5, 12]. Variance F-test and Student’s t-test [33] were applied to assess the results obtained from the proposed method and comparison methods.
Assay of EPL and SPR in Spiked Human Plasma
The proposed method was applied for the estimation of EPL and SPR in spiked human plasma samples to test the ability of the method to estimate both drugs in biological fluids. The peak plasma concentration after oral administration of 100 mg EPL is 1.72 μg/mL [34]. The preparation of plasma samples was carried out as mentioned before and then samples were analyzed. The percentage recovery values were 93.00 to 108.24% as shown in Table 4 and Fig. 5a. The suggested method can also be applied in case of acute toxicity of SPR in patients with impaired hepatic function as it may lead to hepatic coma [35]. The concentrations of SPR could be monitored in plasma after short time of acute toxicity. The percentage recovery values were 89.70 to 108.91% as illustrated in Table 4 and Fig. 5b.
Conclusion
The current study introduces a sensitive and rapid spectrofluorimetric method for the determination of EPL and SPR. The proposed method depends on utilizing N-CQDs as fluorescent probes for the quantitation of the studied drugs depending on the quenching effect of EPL and SPR on the fluorescence emission of N-CQDs without the need for any pre-derivatization steps. Cane sugar and urea were used as starting materials for the rapid microwave-assisted synthesis of the environmentally benign N-CQDs in about 10 min. The water-soluble N-CQDs with an average size of 7.7 nm showed strong fluorescence intensity and high stability. Furthermore, the prepared N-CQDs were applied as nano-sensors for the spectrofluorimetric determination of EPL and SPR in pharmaceutical tablets and spiked human plasma. The quantitation of EPL and SPR has been achieved over a wide range with LOQ of 0.383 µg/mL and 0.262 µg/mL and LOD of 0.126 µg/mL and 0.087 µg/mL. The proposed method has crucial characteristics such as low cost, simplicity, sensitivity and high selectivity.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EPL:
-
Eplerenone
- SPR:
-
Spironolactone
- N-CQDs:
-
Nitrogen doped carbon quantum dots
- CQDs:
-
Carbon quantum dots
- BRB:
-
Britton Robinson Buffer
- IFE:
-
Inner filter effect
References
British Pharmacopoeia (2018) British Pharmacopoeia Commission. The Stationary Office, London, London
Davis KL, Nappi JM (2003) The cardiovascular effects of eplerenone, a selective aldosterone-receptor antagonist. Clin Ther 25(11):2647–2668. https://doi.org/10.1016/s0149-2918(03)80326-0
Shailaja B et al (2015) A rapid assay method development and validation for the estimation of Eplerenone in tablets by UV spectrophotometry. Int J Pharm Pharm Sci 7:327–330
Ganure A et al (2011) Spectrophotometric determination of eplerenone in pharmaceutical bulk and tablet dosage. Der Pharma Chemica 3:566–571
Naina K, Brajesh M, Shashank S (2010) Development and validation of UV-spectrophotometric determination of Eplerenone in bulk and tablets. Int J Pharm Life Sci 1:231–233
Zhang JY, Fast DM, Breau AP (2003) A validated SPE–LC-MS/MS assay for Eplerenone and its hydrolyzed metabolite in human urine. J Pharm Biomed Anal 31:103–115. https://doi.org/10.1016/S0731-7085(02)00595-2
Annapurna MM (2018) New stability indicating liquid chromatographic method for the determination of eplerenone in the presence of internal standard. Asian J Pharm 12:189–194
Gide P, Sonawane S, Chitnis A (2012) Development and validation of RP-HPLC method for estimation of eplerenone in spiked human plasma. J Pharm Anal 2:390–393
Rane VP, Patil KR, Sangshetti JN (2009) Stability-indicating RP-HPLC method for analysis of eplerenone in the bulk drug and in a pharmaceutical dosage form. Acta Chromatogr 21:619–629. https://doi.org/10.1556/achrom.21.2009.4.8
Younis SE, El-Nahass SA, Soliman SA, Youssef RM (2020) Simultaneous micro-determination of eplerenone and torsemide in their combined tablets using HPTLC-dual wavelength spectrodensitometric and spectrophotometric methods. Microchem J 156:104861. https://doi.org/10.1016/j.microc.2020.104861
Belal F, Mabrouk M, Hammad S, Barseem A (2021) A novel eplerenone ecofriendly fluorescent nanosensor based on nitrogen and sulfur-carbon quantum dots. J Fluorescence 31:85–90. https://doi.org/10.1007/s10895-020-02638-4
Prajapati P et al (2016) Quantification of Spironolactone by first and second order UV Derivative Spectrophotometry in bulk and tablet dosage form. Int J Pharm Chem Anal 3:168–173
Hegazy MA, Metwaly FH, Abdelkawy M, Abdelwahab NS (2010) Spectrophotometric and chemometric determination of hydrochlorothiazide and spironolactone in binary mixture in the presence of their impurities and degradants. Drug Test Anal 2:243–251
Millership JS, Parker C, Donnelly D (2005) Ratio spectra derivative spectrophotometry for the determination of furosemide and spironolactone in a capsule formulation. Farmac 60:333–338
Hegazy MA, Metwaly FH, Abdelkawy M, Abdelwahab NS (2011) Validated Chromatographic Methods for Determination of Hydrochlorothiazide and Spironolactone in Pharmaceutical Formulation in Presence of Impurities and Degradants. J Chromatogr Sci 49:129–135. https://doi.org/10.1093/chrsci/49.2.129
Maulik MB, Dadhania K, Faldu S (2012) Development and Validation of RP-HPLC Method for Simultaneous Estimation of Furosemide and Spironolactone in their Combined Tablet Dosage Form. J Pharm Sci Bio-Sci Res 2:144–147
Walash MI, El-Enany N, Eid MI, Fathy ME (2013) Simultaneous determination of metolazone and spironolactone in raw materials, combined tablets and human urine by high performance liquid chromatography. Anal Methods 5:5644–5656. https://doi.org/10.1039/C3AY41110A
Naguib IA et al (2018) Development and validation of HPTLC and green HPLC methods for determination of furosemide, spironolactone and canrenone, in pure forms, tablets and spiked human plasma. Biomed Chromatogr 32:e4304. https://doi.org/10.1002/bmc.4304
Liang J et al (2006) CdSe quantum dots as luminescent probes for spironolactone determination. Talanta 69:126–130
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications: A Review. Chem Soc Res 44:362–381
Kurian M, Paul A (2021) Recent trends in the use of green sources for carbon dot synthesis: a short review. Carbon Trends 3:100032. https://doi.org/10.1016/j.cartre.2021.100032
Molaei MJ (2020) Principles, mechanisms, and application of carbon quantum dots in sensors: a review. Anal Methods 12:1266–1287
Molaei MJ (2019) Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv 9:6460–6481
Wang R, Lu KQ, Tang ZR, Xu YJ (2017) Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis: a review. J Mater Chem A 5:3717–3734
Shaikh SA et al Syntheses of N-Doped Carbon Quantum Dots (NCQDs) from bioderived precursors: a timely update. ACS Sustain Chem Eng 9:3–49. https://doi.org/10.1021/acssuschemeng.0c04727
Liu X et al (2017) N-Doped carbon dots: green and efficient synthesis on a large-scale and their application in fluorescent pH sensing. New J Chem 41:10607–10612. https://doi.org/10.1039/C7NJ01889D
Rurack K (2008) Fluorescence quantum yields: methods of determination and standards. Standardization and quality assurance in fluorescence measurements. Springer, Berlin, pp 101–145
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR (2015) Introduction to Spectroscopy, 5th edn. Cengage learning, USA, pp16–101
Lakowicz JR (1999) Fluorescence Sensing. Principles of fluorescence spectroscopy. Springer, Berlin, pp 531–572
Albani JR (2008) Principles and applications of fluorescence spectroscopy. Wiley, London
Chen S, Yu YL, Wang JH (2018) Inner filter effect-based fluorescent sensing systems: a review. Anal Chim Acta 999:13–26
Guideline ICH (2013) Validation of Analytical Procedures: Text and Methodology, Q2 (R1) Geneva, 2005. In The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry. Pearson Education Limited, Harlow, pp 39–73
Cook CS et al (2003) Pharmacokinetics and metabolism of [14C] eplerenone after oral administration to humans. Drug Metab Dispos 31:1448–1455
https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf. Accessed 16 Sep 2022
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MA Methodology, Investigation, Validation, Writing original draft. NE Conceptualization, Writing-review & editing, Supervision. AM Validation, Writing-review& editing. GH Conceptualization, Writing-review & editing, Supervision. FB Conceptualization, Methodology, Resources, Writing-review and editing, Supervision.
All authors approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethical Approval
There are no human subjects in this research and informed consent is not applicable. The used plasma is pooled plasma from the blood bank of Mansoura university hospital.
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abo Zaid, M.H., El-Enany, N., Mostafa, A.E. et al. Estimation of Two Diuretics Using Fluorescent Nitrogen Doped Carbon Quantum Dots: Application to Spiked Human Plasma and Tablets. J Fluoresc 33, 2209–2218 (2023). https://doi.org/10.1007/s10895-023-03217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03217-z