Abstract
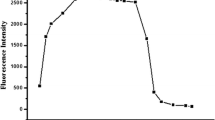
A highly selective, and effective poly(azomethine-urethane)-based chemosensor (HIMA) was prepared, and it used as a fluorescent sensor for the detection of Cr3+ cations in different solutions. The HIMA was prepared in two-step reactions by using hexamethylene diisocyanate, 2,4-dihydroxy benzaldehyde, and 2-aminophenol. The sensitivity and selectivity of the fluorescent probe were tested in the presence of different metal ions. The obtained findings indicated that the chemosensor exhibited a quenching effect against the only Cr3+ ion. The limit of detection (LOD) and limit of quantitation (LOQ) of the chemosensor HIMA were calculated as 7.98 × 10–7 M, and 2.42 × 10–6 M, respectively. In addition, the binding constant (Ka) of the chemosensor was calculated as 5.31 × 105 M−1.








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Ge Q, Feng X, Wang R, Zheng R, Luo S, Duan L, Ji Y, Lin J, Chen H (2020) Mixed redox-couple-involved chalcopyrite phase CuFeS2 quantum dots for highly efficient Cr(VI) removal. Environ Sci Technol 54:8022–8031. https://doi.org/10.1021/acs.est.0c01018
Chen F, Guo S, Wang Y, Ma L, Li B, Song Z, Huang L, Zhang W (2022) Concurrent adsorption and reduction of chromium(VI) to chromium(III) using nitrogen-doped porous carbon adsorbent derived from loofah sponge. Front Environ Sci Eng 16:57. https://doi.org/10.1007/s11783-021-1491-6
Zhang X, Yang Z, Mei J, Hu Q, Chang S, Hong Q, Yang S (2022) Outstanding performance of sulfurated titanomaghemite (Fe2TiO5) for hexavalent chromium removal: Sulfuration promotion mechanism and its application in chromium resource recovery. Chemosphere 287:132360. https://doi.org/10.1016/j.chemosphere.2021.132360
Yang J, Li C, Yang B, Kang S, Zhang Z (2018) Study on adsorption of chromium (VI) by activated carbon from cassava sludge. IOP Conf Ser Earth Environ Sci 128:012017. https://doi.org/10.1088/1755-1315/128/1/012017
Yan BZ, Chen ZF (2019) Influence of pH on Cr(VI) reduction by organic reducing substances from sugarcane molasses. Appl Water Sci 9:61. https://doi.org/10.1007/s13201-019-0940-x
Nguyen NT, Lee SY, Chen SS, Nguyen NC, Chang CT, Hsiao SS, Trang LT, Kao CY, Lin MF, Wang L (2018) Preparation of Zn doped biochar from sewage sludge for chromium ion removal. J Nanosci Nanotechnol 18:5520–5527. https://doi.org/10.1166/jnn.2018.15392
Sangsin S, Srivilai P, Tongraung P (2021) Colorimetric detection of Cr3+ in dietary supplements using a smartphone based on EDTA and tannic acid-modified silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 246:119050. https://doi.org/10.1016/j.saa.2020.119050
Kosla T, Lasocka I, Skibniewska EM, Kolnierzak M, Skibniewski M (2018) Trivalent chromium (Cr III) as a trace element essential for animals and humans. Med Weter 74:560–567. https://doi.org/10.21521/mw.6035
Zarczynska K, Krzebietke S (2018) The effect of chromium on ruminant health. J Elem 25:893–903. https://doi.org/10.5601/jelem.2020.25.1.1963
Lv Z, Tang Y, Dong S, Zhou Q, Cui G (2022) Polyurethane-based polymer electrolytes for lithium batteries: Advances and perspectives. Chem Eng J 430:132659. https://doi.org/10.1016/j.cej.2021.132659
Cheng BX, Gao WC, Ren XM, Ouyang XY, Zhao Y, Zhao H, Wu W, Huang CX, Liu Y, Liu XY, Li HN, Li RKY (2022) A review of microphase separation of polyurethane: Characterization and applications. Polym Test 107:107489. https://doi.org/10.1016/j.polymertesting.2022.107489
Lee DW, Kim HN, Lee DS (2018) Design of azomethine diols for efficient self-healing of strong polyurethane elastomers. Molecules 23:2928. https://doi.org/10.3390/molecules23112928
Kamaci M, Kaya I (2016) New low-band gap polyurethanes containing azomethine bonding: Photophysical, electrochemical, thermal and morphological properties. J Taiwan Inst Chem Eng 59:536–546. https://doi.org/10.1016/j.jtice.2015.08.018
Kaya I, Yildirim M, Kamaci M (2011) A new kind of optical Mn(II) sensor with high selectivity: Melamine based poly(azomethine–urethane). Synth Met 161:2036–2040. https://doi.org/10.1016/j.synthmet.2011.06.029
Kaya I, Kamaci M (2013) Highly selective and stable florescent sensor for Cd(II) based on poly(azomethine-urethane). J Fluoresc 23:115–121. https://doi.org/10.1007/s10895-012-1124-3
Kamaci M, Kaya I (2015) The novel poly(azomethine-urethane): synthesis, morphological properties and application as a fluorescent probe for detection of Zn2+ ions. J Inorg Organomet Polym 25:1250–1259. https://doi.org/10.1007/s10904-015-0234-1
Avci A, Kaya I (2015) A new selective fluorescent sensor for Zn(II) ions based on poly(azomethine-urethane). Tetrahedron Lett 56:1820–1824. https://doi.org/10.1016/j.tetlet.2015.02.079
Kamaci M, Kaya I (2015) 2,4-Diamino-6-hydroxypyrimidine based poly(azomethine-urethane): synthesis and application as a fluorescent probe for detection of Cu2+ in aqueous solution. J Fluoresc 25:1339–1349. https://doi.org/10.1007/s10895-015-1624-z
Kamaci M, Kaya I (2017) A highly selective, sensitive and stable fluorescent chemosensor based on Schiff base and poly(azomethine-urethane) for Fe3+ ions. J Ind Eng Chem 46:234–243. https://doi.org/10.1016/j.jiec.2016.10.035
Kamaci M, Kaya I (2019) Polymeric fluorescent film sensor based on poly(azomethine-urethane): Ion sensing and surface properties. React Funct Polym 136:1–8. https://doi.org/10.1016/j.reactfunctpolym.2018.12.021
Duru Kamaci U, Kamaci M, Peksel A (2019) Poly(azomethine-urethane) and zeolite-based composite: Fluorescent biosensor for DNA detection. Spectrochim Acta A Mol Biomol Spectrosc 212:232–239. https://doi.org/10.1016/j.saa.2019.01.011
Kaya I, Kamaci M (2012) Novel poly(azomethine-urethane)s and their polyphenol derivatives derived from aliphatic diisocyanate compound: synthesis and thermal characterization. J Appl Polym Sci 125:876–887. https://doi.org/10.1002/app.36251
Kaya I, Solak E, Kamaci M (2021) Synthesis and multicolor, photophysical, thermal, and conductivity properties of poly(imine)s. J Taiwan Inst Chem Eng 123:328–337. https://doi.org/10.1016/j.jtice.2021.05.010
Kamaci M, Kaya I (2014) Photophysical, electrochemical, thermal and morphological properties of polyurethanes containing azomethine bonding. J Macromol Sci A 51:805–819. https://doi.org/10.1080/10601325.2014.937129
Kolcu F, Erdener D, Kaya I (2021) Synthesis and characterization of a highly selective turn-on fluorescent chemosensor for Sn2+ derived from diimine Schiff base. Synthetic Met 272:116668. https://doi.org/10.1016/j.synthmet.2020.116668
Feng E, Fan C, Wang N, Liu G, Pu S (2018) A highly selective diarylethene chemosensor for colorimetric detection of CN− and fluorescent relay-detection of Al3+/Cr3+. Dyes Pigm 151:22–27. https://doi.org/10.1016/j.dyepig.2017.12.041
Duru Kamaci U, Kamaci M, Peksel A (2021) A dual responsive colorimetric sensor based on polyazomethine and ascorbic acid for the detection of Al (III) and Fe (II) ions. Spectrochim Acta A Mol Biomol Spectrosc 254:119650. https://doi.org/10.1016/j.saa.2021.119650
Shrivastava A, Gupta VB (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2:21–25. https://doi.org/10.4103/2229-5186.79345
Jofre LG, Cepeda RIC (2016) Effects of Cr3+ ions on electrophysiological parameters of isolated skin of toad Pleurodema thaul. J Adv Pharm Technol Res 7:87–90. https://doi.org/10.4103/2231-4040.184587
Mohanasundaram D, Bhaskar R, Lenin N, Nehru K, Rajagopal G, Kumar GGV, Rajesh J (2022) A simple triphenylamine based turn-off fluorescent sensor for copper (II) ion detection in semi-aqueous solutions. J Photochem Photobiol A 427:113850. https://doi.org/10.1016/j.jphotochem.2022.113850
Chalmardi GB, Tajbakhsh M, Hasani N, Bekhradnia A (2018) A new Schiff-base as fluorescent chemosensor for selective detection of Cr3+: An experimental and theoretical study. Tetrahedron 74:2251–2260. https://doi.org/10.1016/j.tet.2018.03.046
Paul S, Goswami S, Manna A (2015) A differentially selective molecular probe for detection of trivalent ions (Al3+, Cr3+ and Fe3+) upon single excitation in mixed aqueous medium. Dalton Trans 44:11805–11810. https://doi.org/10.1039/C5DT01314C
Mehata MS (2021) An efficient excited-state proton transfer fluorescence quenching based probe (7-hydroxyquinoline) for sensing trivalent cations in aqueous environment. J Mol Liq 326:115379. https://doi.org/10.1016/j.molliq.2021.115379
Hu T, Wang L, Li J, Zhao Y, Cheng J, Li W, Chang Z, Sun C (2021) A new fluorescent sensor L based on fluorene-naphthalene Schiff base for recognition of Al3+ and Cr3+. Inorg Chim Acta 524:120421. https://doi.org/10.1016/j.ica.2021.120421
Krishnan U, Iyer SK (2022) Iminothiophenol Schiff base-based fluorescent probe for dual detection of Hg2+ and Cr3+ ions and its application in real sample analysis. J Photochem Photobiol A Chem 425:113663. https://doi.org/10.1016/j.jphotochem.2021.113663
Das B, Ghosh A, Dorairaj DP, Dolai M, Karvembu R, Mabhai S, Im H, Dey S, Jana A, Misra A (2022) Multiple ion (Al3+, Cr3+, Fe3+, and Cu2+) sensing using a cell-compatible rhodamine-phenolphthalein-derived Schiff-base probe. J Mol Liq 354:118824. https://doi.org/10.1016/j.molliq.2022.118824
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Musa Kamaci is the single author of this manuscript. He has solely designed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The author also declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamaci, M. Poly(Azomethine-urethane)-based Fluorescent Chemosensor for the Detection of Cr3+ Cations in Different Water Samples. J Fluoresc 33, 53–59 (2023). https://doi.org/10.1007/s10895-022-03037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03037-7