Abstract
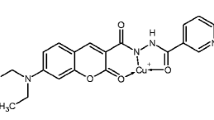
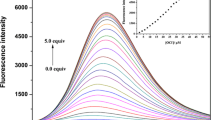
A new fluorescent sensor 1, containing furan and julolidine moieties linked through a Schiff-base, has been synthesized. Distinct “turn-on” fluorescence enhancement of 1 was observed upon the addition of F− in a near-perfect aqueous solution. The binding capabilities of 1 with F− were studied by using fluorescent spectroscopic techniques, ESI-mass analysis and NMR titration measurements. The detection limit for the analysis of F− was found to be 10.02 μM, which is below the WHO guideline (79 μM) for drinking water. Practically, the sensing ability of 1 for F− was successfully applied in real water samples. The sensing mechanism for F− was proposed to be the ICT mechanism via the hydrogen bonding, which was well explained by theoretical calculations.












Similar content being viewed by others
References
Mizukami S, Nagano T, Urano Y, Odani A, Kikuchi K (2002) A fluorescent anion sensor that works in neutral aqueous solution for bioanalytical application. J Am Chem Soc 124:3920–3925
Beer PD, Gale PA (2001) Anion recognition and sensing: the state of the art and future perspectives. Angew Chem Int Ed 40:486–516
Santos-Figueroa LE, Moragues ME, Climent E, Agostini A, Martinez-Manez R, Sancenon F (2013) Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem Soc Rev 42:3489–3613
Lee SA, You GR, Choi YW, Jo HY, Kim AR, Noh I, Kim S-J, Kim Y, Kim C (2014) A new multifunctional Schiff base as a fluorescence sensor for Al3+ and a colorimetric sensor for CN− in aqueous media: an application to bioimaging. Dalton Trans 43:6650–6659
Song EJ, Kim H, Hwang IH, Kim KB, Kim AR, Noh I, Kim C (2014) A single fluorescent chemosensor for multiple target ions: recognition of Zn2+ in 100% aqueous solution and F- in organic solvent. Sensors Actuators B Chem 195:36–43
Park GJ, Hwang IH, Song EJ, Kim H, Kim C (2014) A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 70:2822–2828
Zhou Z, Wang Q, Tan C (2014) Soft matter anion sensing based on lanthanide (Eu 3+ and TB 3+ ) luminescent hydrogels. Soft Mater 12:98–102
Ji X, Yao Y, Li J, Yan X, Huang F (2013) A supramolecular cross-linked conjugated polymer network for multiple fluorescent sensing. J Am Chem Soc 135:74–77
Caballero A, Zapata F, Beer PD (2013) Interlocked host molecules for anion recognition and sensing. Coord Chem Rev 257:2434–2455
Rostami A, Taylor MS (2012) Polymers for anion recognition and sensing. Macromol Rapid Commun 33:21–34
Browne D, Whelton H, O’Mullane D (2005) Fluoride metabolism and fluorosis. J Dent 33:177–186
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, Guten U, Werli B (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077
Jagtap S, Yenkie MK, Labhsetwar N, Rayalu S (2012) Fluoride in drinking water and Defluoridation of water. Chem Rev 112:2454–2466
Graham N (1999) Guidelines for drinking-water quality, 2nd edition, addendum to volume 1 – recommendations, World Health Organisation, Geneva, 1998, 36 pages. Urban Water 1:183
Ryu HH, Lee YJ, Kim SE, Jo TG, Kim C (2016) A colorimetric F− chemosensor with high selectivity: experimental and theoretical studies. J Incl Phenom Macrocycl Chem 86:111–119
Lee JH, Lee SH, So YA, Park GJ, Kim C (2015) Simultaneous detection of F − and CN − by a simple colorimetric Chemosensor with high selectivity. Bull Kor Chem Soc 36:1618–1624
Lee HJ, Park SJ, Sin HJ, Na YJ, Kim C (2015) A selective colorimetric chemosensor with an electron-withdrawing group for multi-analytes CN − and F −. New J Chem 39:3900–3907
Lee M, Jo S, Lee D, Xu Z, Yoon J (2015) A new naphthalimide derivative as a selective fluorescent and colorimetric sensor for fluoride, cyanide and CO2. Dyes Pigments 120:288–292
Cho J, Kim I, Moon JH, Jung HS, Kim JS (2016) Triazolium-promoted highly selective fluorescence “turn-on” detection of fluoride ions. Dyes Pigments 132:248–254
Park JJ, Kim Y-H, Kim C, Kang J (2011) Naked eye detection of fluoride and pyrophosphate with an anion receptor utilizing anthracene and nitrophenyl group as signaling group. Tetrahedron Lett 52:2759–2763
Kang J, Lee YJ, Lee SK, Park JJ, Kim Y, Kim SJ, Kim C (2010) A naked-eye detection of fluoride with urea/thiourea receptors which have both a benzophenone group and a nitrophenyl group as a signalling group. Supramol Chem 22:267–273
Yoo M, Park S, Kim H-J (2016) Activatable colorimetric and fluorogenic probe for fluoride detection by oxazoloindole-to-hydroxyethylindolium transformation. RSC Adv 6:19910–19915
Rao KS, Balaji T, Rao TP, Babu Y, Naidu GRK (2002) Determination of iron, cobalt, nickel, manganese, zinc, copper, cadmium and lead in human hair by inductively coupled plasma- atomic emission spectrometry. Spectrochim Acta Part B-atomic Spectrosc 57:1333–1338
Sturgeon RE, Berman SS, Desaulniers A, Russell DS (1979) Determination of iron, manganese, and zinc in seawater by graphite furnace atomic absorption spectrometry. Anal Chem 51:2364–2369
Gulaboski R, Mireski V, Scholz F (2002) An electrochemical method for determination of the standard Gibbs energy of anion transfer between water and n-octanol. Electrochem Commun 4:277–283
Montoya LA, Pluth MD (2012) ChemComm selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells w. 4767-4769
Park GJ, Jo HY, Ryu KY, Kim C (2014) A new coumarin-based chromogenic chemosensor for the detection of dual analytes al 3+ and F −. RSC Adv 4:63882–63890
Sen B, Mukherjee M, Banerjee S, Chattopadhyay P (2015) A rhodamine-based “turn-on” al 3+ ion-selective reporter and the resultant complex as a secondary sensor for F − ion are applicable to living cell staining. Dalton Trans 44:8708–8717
Datta BK, Thiyagarajan D, Ramesh A, Das G (2015) A sole multi-analyte receptor responds with three distinct fluorescence signals: traffic signal like sensing of Al3+, Zn2+ and F−. Dalton Trans 44:13093–13099
Ghosh K, Panja S, Sarkar T (2015) Rhodamine-linked pyridyl thiourea as a receptor for selective recognition of F−, Al3+ and ag+ under different conditions. Supramol Chem 27:490–500
Kim YS, Lee JJ, Choi YW, Kim C (2016) Simultaneous bioimaging recognition of cation Al3+ and anion F− by a fluorogenic method. Dyes Pigments 129:43–53
Liu H, Zhang B, Tan C, Jiang Y (2016) Simultaneous bioimaging recognition of Al3+ and Cu2+ in living-cell, and further detection of F− and S2− by a simple fluorogenic benzimidazole-based chemosensor. Talanta 161:309–319
Lee JJ, Park GJ, Kim YS, Lee SY, Lee HJ, Noh I, Kim C (2015) A water-soluble carboxylic-functionalized chemosensor for detecting Al3+ in aqueous media and living cells: experimental and theoretical studies. Biosens Bioelectron 69:226–229
Choi YW, Park GJ, Na YJ, Jo HY, Lee SA, You GR, Kim C (2014) A single schiff base molecule for recognizing multiple metal ions: a fluorescence sensor for Zn(II) and al(III) and colorimetric sensor for Fe(II) and Fe(III). Sensors Actuators B Chem 194:343–352
Jang YK, Nam UC, Kwon HL, Hwang IH, Kim C (2013) A selective colorimetric and fluorescent chemosensor based-on naphthol for detection of Al3+ and Cu2+. Dyes Pigments 99:6–13
Kim KB, Kim H, Song EJ, Kim S, Noh I, Kim C (2013) A cap-type Schiff base acting as a fluorescence sensor for zinc(II) and a colorimetric sensor for iron(II), copper(II), and zinc(II) in aqueous media. Dalton Trans 42:16569–16577
Yang C, Gong D, Wang X, Deng Y, Guo Y (2016) A new highly copper-selective fluorescence enhancement chemosensor based on BODIPY excitable with visible light and its imaging in living cells. Sensors Actuators B Chem 224:110–117
Boonkitpatarakul K, Wang J, Niamnont N, Mcdonald L, Pang Y, Sukwattanasinitt M (2016) Novel turn-on fluorescent sensors with mega stokes shifts for dual detection of Al3+ and Zn2+. ACS Sensors 1:144–150
Hu Y, Ke Q, Yan C, Huang XH, Hu S (2016) A new fluorescence chemosensor for selective detection of copper ion in aqueous solution. Tetrahedron Lett 57:2239–2243
Choi YW, You GR, Lee JJ, Kim C (2016) Turn-on fluorescent chemosensor for selective detection of Zn2+ in an aqueous solution: experimental and theoretical studies. Inorg Chem Commun 63:35–38
Lee SY, Bok KH, Kim JA, Kim C (2016) Simultaneous detection of Cu2+ and Cr3+ by a simple Schiff-base colorimetric chemosensor bearing NBD (7-nitrobenzo-2-oxa-1,3-diazolyl) and julolidine moieties. Tetrahedron 72:5563–5570
Jo HY, Lee SA, Na YJ, Kim C (2015) A colorimetric Schiff base chemosensor for CN− by naked-eye in aqueous solution. Inorg Chem Commun 54:73–76
Lee JJ, Park GJ, Choi YW, Kim C (2015) Detection of multiple analytes (CN− and F−) based on a simple pyrazine-derived chemosensor in aqueous solution: experimental and theoretical approaches. Sensors Actuators B Chem 207:123–132
Puthiyedath T, Bahulayan D (2017) A click-generated triazole tethered oxazolone-pyrimidinone dyad: a highly selective colorimetric and ratiometric FRET based fluorescent probe for sensing azide ions. Sensors Actuators B Chem 239:1076–1086
Kawanishi Y, Kikuchi K, Takakusa H, Nagano T (2000) Design and Synthesis of intramolecular resonance-energy transfer probes for use in Ratiometric measurements in aqueous solution. Angew Chem 39:3438–3440
Becke AD (1993) Density-functional thermochemistry. III The Role of Exact Exchange J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J.M.Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Peters CG and JAP (2004) GAUSSIAN 03 (revision B.02). Gaussian, Inc., Wallingford
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Francl MM, Pietro WJ, Hehre WJ, Binkely JS, Gordon MS, Defrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. 23. A polarization-type basis set for 2nd-row elements. J Chem Phys 77:3654–3665
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Cossi M, Barone V (2001) Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys 115:4708–4717
O’Boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Job P (1928) Formation and stability of inorganic complexes in solution. Ann Chim 9:113–203
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Tsui Y-K, Devaraj S, Yen Y-P (2012) Azo dyes featuring with nitrobenzoxadiazole (NBD) unit: a new selective chromogenic and fluorogenic sensor for cyanide ion. Sensors Actuators B Chem 161:510–519
Acknowledgements
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2014R1A2A1A11051794 and NRF-2015R1A2A2A09001301) are gratefully acknowledged. This subject is supported by Korea Ministry of Environment (MOE) as "The Chemical Accident Prevention Technology Development Project". We thank Nano-Inorganic Laboratory, Department of Nano & Bio Chemistry, Kookmin University to access the Gaussian 03 program packages.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 148 kb)
Rights and permissions
About this article
Cite this article
Jeong, H.Y., Lee, S.Y. & Kim, C. Furan and Julolidine-Based “Turn-on” Fluorescence Chemosensor for Detection of F− in a Near-Perfect Aqueous Solution. J Fluoresc 27, 1457–1466 (2017). https://doi.org/10.1007/s10895-017-2085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2085-3