Abstract
A green, simple, quick and economical method is implemented for the first time for the simultaneous estimation of cetirizine (CTZ) and azelastine (AZE) as co-administered eye drops. The method relies on synchronous spectrofluorimetry with ∆λ = 60 nm. Cetirizine can be estimated at 231 nm and AZE can be measured at 294 nm, each at the other’s zero crossing point. All factors affecting the method were studied and properly optimized. Good correlation was obtained in the range of 0.1–2 µg mL−1 for both drugs. The limits of detection were 0.014 and 0.010 µg mL−1 and limits of quantitation were 0.043 and 0.029 µg mL−1 for CTZ and AZE, respectively. Moreover, ICH guidelines were carried out to validate the adopted method. The method was suitable for the analysis of CTZ and AZE in synthetic mixtures, eye drops and aqueous humor. The mean percentage of recoveries of CTZ and AZE in spiked aqueous humor were 99.83 and 99.37, respectively. Furthermore, Green Analytical Procedure Index (GAPI) and analytical Eco-scale approaches were used to evaluate the greenness of the suggested method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocular allergy disease has become more prevalent during the last several decades as it affects 40% of the population globally [1]. It causes significant decline in the work and educational productivity as well as the overall quality of life [2]. Seasonal and perennial allergic conjunctivitis are the most common types of ocular allergy, in which the conjunctiva of the eye becomes inflamed as a result of an immunoglobulin E-mediated hypersensitivity reaction [3]. Ocular allergy results from pollen, molds and dust mites leading to watery eyes, itching, irritated eye and chemosis [4].
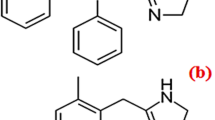
CTZ is [2-[4-[(4-Chlorophenyl) phenylmethyl]-1–piperazinyl] ethoxy acetic acid) (Fig. 1a) [5]. It is histamine (H1)-receptor antagonist used in ocular allergy [6]. BP [5] recommended non aqueous potentiometric titration using sodium hydroxide while USP [7] stated HPLC for CTZ assay. There are several documented methods for its assay either in dosage form or human plasma as HPLC [8,9,10,11,12], capillary electrophoresis [13, 14], spectrofluorimetry [15,16,17,18] and spectrophotometry [19, 20].
AZE is 4-[(4-chlorophenyl)methyl]-2-(1-methylazepanyl)-1-phthalazinone (Fig. 1b) [5]. It is mast cell stabilizer that is used topically to relief allergic conjunctivitis [21]. Both BP [5] and USP [7] recommended non aqueous potentiometric titration with perchloric acid for AZE assay. Many articles have been published for its assay as HPLC [22,23,24,25,26], HPTLC [27, 28], capillary electrophoresis [29], electrochemical analysis [30, 31], NMR [32], spectrofluorimetry [33,34,35] and spectrophotometry [36,37,38].
Topical antihistamine and mast cell stabilizer are commonly co-administered for treatment of eye allergy [39]. CTZ as an antihistamine provides relief from symptoms before AZE starts working. AZE inhibits degranulation and the release of histamine. So, the effect of cetirizine in eye allergy can be enhanced by the addition of AZE [39].
To date, there is no documented method for the simultaneous determination of CTZ and AZE drug co-administration in aqueous humor. So, there is a need to establish a reliable new procedure for their assay in aqueous humor for therapeutic drug monitoring. The normal emission fluorescence spectra of the two studied drugs are severely overlapped when applying conventional spectrofluorimetric approach. Therefore, we applied synchronous fluorescence spectroscopy (SFS) to solve such problem and analyze each drug in the presence of the other in aqueous humor.
In constant wavelength SFS, both excitation and emission monochromators are simultaneously scanned at constant scan rates and a constant wavelength interval (∆λ) is kept between excitation and emission wavelengths. In conventional fluorescence, the intensity of fluorescence emission relies on emission wavelength while in SFS, it relies on ∆λ (both excitation and emission wavelengths). For constant wavelength SFS, the fluorescence intensity can be expressed as:
Where c is the concentration of analyte, l is the path length of light and k is an experimental constant. For a given set of experimental conditions, fluorescence intensity is proportional to the concentration of the analyte. SFS is useful in the analysis of mixtures due to its apparent advantages including high selectivity, low scattering light interference, simple spectra and quick measurement in a single run [40].
Accordingly, a reproducible, sensitive, cheap, easy and eco-friendly SFS procedure was studied for the assay of CTZ and AZE simultaneously in their synthetic mixtures, aqueous humor as well as in their single eye drops. Assessment of the adopted method greenness was also performed using GAPI and analytical Eco-scale tools.
Experimental
Materials and Reagents
-
CTZ and AZE pure samples (99.95% and 99.80% purity as labeled, respectively) were supplied by Apex Co. (Cairo) and European Egyptian Pharmaceuticals Industry (Alexandria), respectively.
-
Ophthalmic formulations: Cetirizine® eye drops 1%, a product of Pharo Pharma (batch no. 5669002). Azelast® eye drops 0.05% manufactured by The Tenth of Ramadan for Pharmaceutical industries and diagnostic (RAMEDA) (for Hikma Pharmaceutical industries) (batch no. 202792).
-
HPLC organic solvents were from Sigma Aldrich (Germany).
-
Acetic acid, phosphoric acid, boric acid, sodium hydroxide, hydrochloric acid, sulfuric acid, nitric acid, tween 80, sodium dodecyl sulphate, β-cyclodextrin, carboxy methyl cellulose and cetrimide were all attained from El-Nasr Pharmaceutical Chemicals Co. (Cairo, Egypt).
-
The water utilized throughout the experiment was double distilled water.
Instruments
A Cary Eclipse spectrofluorometer with a xenon lamp and 5 mm slits was utilized. The synchronous mode was set at ∆λ = 60 nm and smoothing factor = 20. A Sonix IV model-SS101H 230 (USA) was utilized. A Consort pH meter was utilized for pH adjustment.
Preparation of Standard Solutions
In 100 mL calibrated flasks, standard stock solutions (100 µg mL−1) of both CTZ and AZE were prepared separately by dissolving 10.0 mg of each in 100 mL methanol. Then, dilution was made to have standard working solutions of both drugs (10 µg mL−1) in methanol. All solutions were wrapped in aluminum foil [5] and kept in the refrigerator.
Procedures
Construction of the Calibration Curves
In 10 mL calibrated flasks, appropriate volumes of CTZ and AZE standard working solutions were transferred separately to get final concentration ranges (0.1–2 µg mL−1) for both drugs. Then 1 mL 0.4 M H2SO4 was added followed by dilution to 10 mL with water and mixed well. A blank experiment was carried out in the same manner to obtain the relative synchronous fluorescence intensity (RSFI). The synchronous fluorescence spectra were measured at ∆λ = 60 nm. CTZ and AZE spectra were measured at 231 and 294 nm, respectively. Besides, RSFI were plotted versus the ultimate drugs concentrations (µg mL−1) and the regression equations were computed.
Analysis of CTZ/AZE in Laboratory Prepared Mixtures
Laboratory-prepared mixtures of CTZ and AZE in different ratios were prepared from the standard working solutions in 10 mL calibrated flasks. The procedure under ‘Construction of the calibration curves’ was carried out.
Analysis of CTZ/AZE in their Ophthalmic Formulations
For Cetirizine®: one milliliter was taken from the formulation into 100 mL flask and the volume was completed with methanol. Then, five milliliters were transferred from the preceding solution into 50 mL flask (10 µg mL−1).
For Azelast®: one milliliter was taken into 50 mL flask and the volume was completed with methanol (10 µg mL−1). Samples in the linearity range were taken and the procedure described under ‘Construction of calibration curves’ was applied to calculate the content of the eye drops from the regression equations.
Analysis of CTZ/AZE in Aqueous Humor
Artificial aqueous humor was prepared in the lab to simulate the natural one of that of the human [41]. In 10 mL calibrated flasks, one milliliter of the artificial aqueous humor was transferred. Subsequently, different volumes of both CTZ and AZE working standard solutions containing (1.0–20 µg) were added. Implement the steps mentioned in ‘Construction of the calibration curves’.
Results and Discussion
Spectral Characteristics
Both CTZ and AZE were stated to exhibit innate fluorescence. CTZ was estimated by conventional spectrofluorimetry in acidic medium using water as the diluting solvent at 235/294 nm [18]. Also, AZE was estimated in water at 286/364 nm [34]. As shown in Fig. 2, it is noticed that the spectra of both drugs suffered from overlapping. To solve this problem, SFS mode was operated which resulted in a good separation of the two spectra permitting their assay in aqueous humor simultaneously. As shown in Fig. 3, CTZ could be estimated at 231 nm in presence of AZE, and AZE at 294 nm in presence of CTZ.
Table 1 showed a comparison of the adopted method to the published work for the assay of each drug.
Optimization of SFS factors
Selection of Optimal Δ λ
The value of ∆λ is important in SFS as it affects peak resolution and sensitivity. Firstly, different ∆λ values (20–140 nm) were tested. From the study, ∆λ = 60 nm was the most suitable as it provided better peak shape and sensitivity for both drugs. Lower ∆λ values than 60 nm led to lower fluorescence intensity and higher ∆λ values than 60 nm led to poor separation of the two spectra.
Impact of pH
Briton Robinson buffer with pH range (2–12), 0.2 M H2SO4 and 0.1 M NaOH were used to study the impact of pH on the RSFI. Both CTZ and AZE has nitrogen atom in their structures which could be protonated in acidic solutions. The protonation has shown to significantly increase fluorescence intensity [18, 42]. This may be the reason behind the increased RSFI of both drugs in more acidic solutions and decreased in basic solutions. In more basic solutions (0.1 M NaOH), RSFI decreased significantly which may be attributable to their degradation. However, RSFI enhanced in 0.2 M H2SO4 for both drugs especially CTZ (Fig. 4a). So, H2SO4 was chosen in the study to increase the sensitivity for both drugs.
Impact of Type and Concentration of Acid
Different acids were tried in the study. These acids were: nitric acid, acetic acid, phosphoric acid, hydrochloric acid and sulfuric acid. As shown in Fig. 4b, sulfuric acid was the most suitable one as it gave high RSFI for both compounds. Furthermore, the influence of H2SO4 concentration on RSFI of CTZ and AZE was studied. The results showed that increasing the molarity up to 0.3 M led to eventual increase in RSFI of CTZ, molarities 0.4 M and 0.5 M gave no increase in RSFI than 0.3 M. For AZE, H2SO4 concentration (0.05–0.5 M) had no significant effect on RSFI. So, H2SO4 concentration of 0.4 M was chosen in the SFS.
Impact of Surfactants
Different surfactants were utilized to check their impact on the RSFI (1% w/v of each). All the studied surfactants (carboxymethyl cellulose, cetrimide, sodium dodecyl sulphate, β-cyclodextrin, tween 80) led to decrease in RSFI for the two compounds when compared to using 0.4 M H2SO4. Hence, the study was continued without surfactant.
Impact of Diluting Solvents
Various solvent systems were attempted to determine the best conditions such as fluorescence intensity, stability and separation of the two spectra. These solvents were: water, methanol, ethanol, acetonitrile, isopropanol, butanol, dimethyl formamide and acetone. Butanol, isopropanol and dimethyl formamide gave high blank readings for both compounds. Acetone completely quenched the fluorescence intensity of the two drugs. Methanol, ethanol and acetonitrile caused shift in the maximum wavelength of CTZ and interference was noticed between the two compounds. Water was found to be the optimal one as it provided the best sensitivity and spectra separation. Also, it adds advantages to the procedure to be ecofriendly and cost effective without utilizing organic solvents.
Impact of Time
Time effect on the RSFI of CTZ and AZE was also studied. RFSI occurred immediately and remained stable for two hours.
Validation of the Proposed Method
To confirm that the suggested SFS method is suitable for its intended use, the ICH Guidelines [43] were followed.
Linearity
A linear relationship was obtained between RSFI values and their corresponding drug concentrations (µg mL−1) over the range 0.1–2 µg mL−1 for both drugs. Linear analysis was made and the resulted regression equations were as following:
Where C is the concentration of the drug in µg mL−1. The calculated analytical parameters are presented in Table 2.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD and LOQ were calculated in Table 2 pursuant to ICH Guidelines [43] by applying the following equations:
LOD = 3.3 σ/S and LOQ = 10 σ/S. Where σ: Standard deviation of the intercept. S: the slope of the regression line. LOD and LOQ values indicate the high sensitivity of the suggested method.
Accuracy and Precision
The accuracy was confirmed from the accepted percentage recoveries in Table 3. Also, the results were compared with those of the comparison methods [18, 34] and it was found that there is no remarkable difference between the methods. Intraday precision was performed by estimating three varied concentrations within the linearity range three times in the same day. But, interday precision was performed in three different days. The accepted % RSD values (Table 4) prove the precision.
Selectivity
The SFS procedure was used for the synchronized estimation of CTZ and AZE in synthetic combinations composed of different ratios of the two drugs as shown in Fig. 5. CTZ was measured at 231 nm where AZE shows no interference. Similarly, AZE was measured at 294 nm without any interference from the other drug. Upon measuring the peak amplitude of each drug, the corresponding drug concentrations were calculated from the regression equation. Satisfactory results were obtained and mentioned in Table 5. Furthermore, the method selectivity was achieved by the estimation of the studied drugs in aqueous humor. The prepared artificial aqueous humor composed of sodium chloride, potassium chloride, dibasic sodium phosphate, sodium bicarbonate, calcium chloride, potassium chloride dihydrate, magnesium chloride hexahydrate, dibasic sodium phosphate, sodium bicarbonate, dextrose, glutathione disulfide, hydrochloric acid and/or sodium hydroxide and water for injection [41]. None of these components interfered with the analysis of the two compounds as proved by the high percentage recoveries and the small values of SD for CTZ and AZE assay in aqueous humor (Table 6 and Fig. 6).
Robustness
Assessment of the suggested method robustness was performed by applying premeditated slight variation in the concentration of H2SO4 (0.4 M ± 0.1). These variations had negligible influence on RSFI which reflects the robustness.
Applications
Assay of CTZ/AZE Synthetic Mixtures
The suggested procedure was implemented for the estimation of the investigated compounds in their synthetic mixtures (Fig. 5). The accepted %recoveries reflect the accuracy (Table 5).
Assay of CTZ and AZE in their Ophthalmic Formulations
This synchronous spectrofluorimetry was utilized to estimate CTZ and AZE directly in their ophthalmic formulations for extending its utilization in quality control laboratories. The results attained were contrasted with those of the comparison procedures as presented in Table 7. Statistical assessment of the results utilizing F and t tests demonstrated that there is no remarkable difference between the two procedures [44].
Assay of CTZ and AZE in Aqueous Humor
The suggested procedure enables the determination of CTZ and AZE simultaneously in aqueous humor (Fig. 6). Data in Table 6 revealed that the mean absolute recoveries and % RSD of CTZ and AZE in aqueous humor were 99.83 ± 1.95 and 99.37 ± 0.69, respectively.
Assessment of the Greenness of the Proposed Method
Several analytical tools are currently present to assess the methodologies concerning their ecological impact. GAPI and analytical Eco-scale were conducted in this study. The viewable presentation of GAPI (five pentagram) makes it easy to select the greenest approach for a definite study [45]. It is a semi-quantitative tool that gives exhaustive information on the evaluated practices through providing a more detailed evaluation for each step of the analytical methodology from sample collection to final determination. GAPI assessment tool of the studied procedure is shown in Fig. 7.
The green assessment profile for the proposed SFS using the GAPI tool [45]
Analytical Eco-scale [46] is another greenness assessment tool. It determines penalty points for various factors involved in the study. Then, the total score is subtracted from the ideal value 100. The suggested procedure is found to be an excellent green one (Table 8).
Conclusion
In severe ocular allergies, a combination of cetirizine and azelastine eye drops could be recommended. A green, easy and quick synchronous fluorescence approach was studied for the first time to estimate both drugs simultaneously in aqueous humor for clinical assessment. The method was simple and did not require the use of expensive equipment or solvents. The method was subjected to ICH guidelines and characterized by wide linearity range, accuracy, precision, selectivity and robustness. Furthermore, it could be useful for analyzing the cited drugs in their ophthalmic formulations in quality control laboratories.
Data Availability
All the data and the materials are available all-over the study.
Code Availability
No software or any computational study was not used for this study.
References
Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P (2020) ICON: diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol 124:118–134
Miyazaki D, Fukagawa K, Okamoto S, Fukushima A, Uchio E, Ebihara N, Shoji J, Namba K, Shimizu Y (2020) Eidemiological aspects of allergic conjunctivitis. Allergol Int 69:487–495
Jeremy Ono S, Abelson MB (2005) Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol 115:118–122
Bielory L, Friedlaender MH (2008) Allergic Conjunctivitis. Immunol Allergy Clin North Am 28:43–58
British Pharmacopoeia (2017) The Stationary Office: electronic version Volume I & II, London.
Malhotra RP, Meier E, Torkildsen G, Gomes PJ, Jasek MC (2019) Safety of cetirizine ophthalmic solution 0.24% for the treatment of allergic conjunctivitis in adult and pediatric subjects. Clin Ophthalmol 13:403–413
The United States Pharmacopoeia (2017) 40th edn. and National Formulary 35th edn. U.S. Pharmacopoeial Convention, Rockville.
Ma M, Feng F, Sheng Y, Cui S, Liu H (2007) Development and evaluation of an efficient HPLC/MS/MS method for the simultaneous determination of pseudoephedrine and cetirizine in human plasma: Application to Phase-I pharmacokinetic stud. J Chromatogr B 846:105–111
Souri E, Hatami A, Ravari NS, Alvandifar F, Tehrani MB (2013) Validating a stability indicating HPLC method for kinetic study of cetirizine degradation in acidic and oxidative conditions. Iran J Pharm Res 12:287–294
Aly FA, EL-Enany N, Elmansi H, Nabil AA (2017) Validated reversed phase hplc method for simultaneous determination of the antihistaminic cetirizine and beta2-adrenergic agonist salbutamol in their co-formulated tablets. SM anal bioanal technique 2
Shamshad H, Mirza AZ (2021) Application of RP-HPLC method for the simultaneous determination of cetirizine in the presence of quinolones. Future J Pharm Sci 7:1–6
Shamshad H, Sayqal A, Zeb J, Mirza AZ (2021) Simultaneous determination of chloroquine and pyrimethamine with cetirizine in an active form and human serum by RP-HPLC. J Chromatogr Sci bmab018
Uysal UD, Tunçel M (2006) Validated capillary electrophoresis study for the determination of cetirizine in pharmaceutical forms. J Liq Chromatogr Relat Technol 29:1781–1792
Javid FS, Shafaat A, Zarghi A (2014) Determination of cetirizine and its impurities in bulk and tablet formulation using a validated capillary zone electrophoretic method. J Anal Chem 69:442–447
Ibrahim F, El-Din MS, Eid M, Wahba MEK (2011) Spectrofluorimetric determination of some H1 receptor antagonist drugs in pharmaceutical formulations and biological fluids. Int J Pharm Sci and Res 2:2056–2072
Wei XL, Lei XR, Gong Q, Wang LS, Liao Y (2011) Determination of cetirizine dihydrochloride by anti-fluorescence quenching on rhodamine B-Sodium tetraphenylborate system. Guang Pu Xue Yu Guang Pu Fen Xi 31:1596–1600
El-Din MKS, Ibrahim F, Eid MI, Wahba MEK (2012) Validated spectroflurimetric determination of some H1 receptor antagonist drugs in pharmaceutical preparations through charge transfer complexation. J Fluoresc 22:175–191
El-Kommos ME, El-Gizawy SM, Atia NN, Hosny NM (2015) Determination of some non-sedating antihistamines via their native fluorescence and derivation of some quantitative fluorescence intensity - structure relationships. J Fluoresc 25:1695–1709
Pourghazi K, Khoshhesab ZM, Golpayeganizadeh A, Shapouri MR, Afrouzi H (2011) Spectrophotometric determination of cetirizine and montelukast in prepared formulations. Int J Pharm Pharm Sci 3:128–130
El-Didamony AM, Ramadan GM (2020) Charge-transfer interaction between antihistamine antiallergic drugs, diphenhydramine, fexofenadine, cetirizine and two π-acceptors in pharmaceutical forms. SN Appl Sci 2:1–14
Lambiase A, Micera A, Bonini S (2009) Multiple action agents and the eye: do they really stabilize mast cells? Curr Opin Allergy Clin Immunol 9:454–465
El-Shaheny RN, Yamada K (2014) Stability study of the antihistamine drug azelastine HCl along with a kinetic investigation and the identification of new degradation products. Anal Sci 30:691–697
Hassouna M, Abdelrahman M, Abdelfatah M (2017) Simultaneous determination of azelastine hydrochloride and benzalkonium chloride by RP-HPLC method in their ophthalmic solution. J Forensic Sci Crim Investig 1:555–565
Patel S, Pasha TY (2018) Stability-indicating high-performance liquid chromatography method for determination of antihistamine drug azelastine. Asian J Pharm Clin Res 11:248–251
El-Masry AA, Hammouda MEA, El-Wasseef DR, El-Ashry SM (2019) Eco-friendly green liquid chromatographic determination of azelastine in the presence of its degradation products: Applications to degradation kinetics. J AOAC Int 102:81–90
El-Masry AA, Hammouda ME, El-Wasseef DR, El-Ashry SM (2020) Eco-friendly green liquid chromatographic separations of a novel combination of azelastine and fluticasone in the presence of their pharmaceutical dosage form additives. Curr Anal Chem 16:277–286
Salama NN, Abdel-Razeq SA, Abdel-Atty S, El-Kosy N (2014) Development and validation of densitometry TLC stability indicating method for quantitative determination of azelastine hydrochloride and emedastine difumarate in their drug products. J Pharm Res Int 4:79–92
Patel KG, Patel SKG, Shah PA, Tandel DB, Gandhi TR (2020) Development and validation of HPTLC method along with forced degradation study for the simultaneous estimation of azelastine hydrochloride and fluticasone propionate in nasal spray formulation using design of experiment approach. Indian J Pharm Educ Res 54:155–165
Adelwahab NS, Farid NF, Elagawany M, Abdelmomen EH (2018) Efficient UPLC and CE methods for the simultaneous determination of azelastine hydrochloride and its genotoxic impurity. Biomed Chromatogr 32:e4346
Abdel-Razeq SA, Foaud MM, Salama NN, Abdel-Atty S, El- Kosy N (2011) Voltammetric determination of azelastine-HCl and emedastine difumarate in micellar solution at glassy carbon and carbon paste electrodes. Sensing in Electroanalysis 6:289–305
Elghobashy MR, Badran OM, Salem MY, Kelani KM (2013) Application of membrane selective electrodes for the determination of azelastine hydrochloride in the presence of its alkaline degradant in eye drops and plasma. J Anal Bioanal Electrochem 5:325–340
El-Masry AA, El-Wasseef DR, Eid M, Shehata IA, Zeid AM (2021) Quantitative proton nuclear magnetic resonance method for simultaneous analysis of fluticasone propionate and azelastine hydrochloride in nasal spray formulation. R Soc Open Sci 8:210483
El-Masry AA, Hammouda MEA, El-Wasseef DR, El-Ashry SM (2017) Validated sensitive spectrofluorimetric method for determination of antihistaminic drug azelastine HCl in pure form and in pharmaceutical dosage forms: application to stability study. Luminescence 32:177–181
Ragab MAA, El-Kimary EI (2018) Investigation of the spectrofluorimetric behavior of azelastine and nepafenac: Determination in ophthalmic dosage forms. Spectrochim Acta A 204:260–266
Shekhar S, Bali A (2021) Spectrofluorimetric method for the determination of azelastine hydrochloride in bulk and nasal formulations. J Appl Spectrosc 88:674–680
Gouda AA, El Sheikh R, El Saied H (2015) Extractive spectrophotometric determination of azelastine hydrochloride in pure form and pharmaceutical formulations. Can Chem Trans 3:29–41
Hassouna MEM, Abdelrahman MM, Mohamed MA (2017) Determination of azelastine hydrochloride and benzalkonium chloride in their ophthalmic solution by different spectrophotometric methods. World J Appl Chem 2:48–56
El-Masry AA, Hammouda MEA, El-Wasseef DR, El-Ashry SM (2018) Validated spectroscopic methods for determination of anti-histaminic drug azelastine in pure form: Analytical application for quality control of its pharmaceutical reparations. Spectrochim Acta A 191:413–420
Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara‐Blanco A (2015) Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst Rev 6
Eiroa A, De-Armas G, Estela J, Cerda V (2010) Critical approach to synchronous spectrofluorimetry. I Trends Anal Chem 29:885–901
Macri A, Marini V, Sangalli G, Fucile C, Iester M, Mattioli F (2015) An artificial aqueous humor as a standard matrix to assess drug concentration in the anterior chamber by high performance liquid chromatography methods. Clin Lab 61:47–52
Ohannesian L (2002) Handbook of pharmaceutical analysis. Marcel Dekker, New York
ICH Harmonised Tripartite Guidelines (2005) Validation of analytical procedures: text and methodology Q2(R1). http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 29 August 2021
Miller J, Miller JC (2018) Statistics and Chemometrics for Analytical Chemistry. Pearson Education Limited, London
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 181:204–209
Gałuszka A, Konieczka P, Migaszewski ZM, Namies’nik J (2012) Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal Chem 37:61–72
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
WNAA: methodology, formal analysis, validation, investigation, writing–original draft. FAA: conceptualization, validation, writing–review & editing, resources, supervision. ZAS: validation, writing–review & editing, supervision. SS: validation, writing–review & editing, supervision.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd-AlGhafar, W.N., Aly, F.A., Sheribah, Z.A. et al. Synchronous Fluorescence as a Green and Selective Method for the Simultaneous Determination of Cetirizine and Azelastine in Aqueous Humor. J Fluoresc 32, 1199–1210 (2022). https://doi.org/10.1007/s10895-022-02913-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02913-6