Abstract
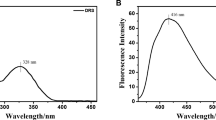
A simple synchronous fluorescent chemosensor 3-hydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one (3-HC) has been synthesized for the selective analysis of Al3+. On the addition of Al3+, 3-HC displayed a redshift with a change in wavelength of emission maximum from 436 to 465 nm along with enhancement in fluorescence intensity, which formed the basis for its sensitive detection. Under optimized conditions, 3-HC was applied for the determination of Al3+ in the concentration range of 1 × 10–7-1 × 10–6 M. The limit of detection (LOD) and limit of quantification (LOQ) values were found out to be 1.69 × 10–8 and 5.07 × 10–8 M respectively. Further, the developed method was applied for the analysis of Al3+ in real water samples (tap water, bottled water, and tube well water) which showed good recovery values in the range of 95–99.7% with RSD less than 4%.






Similar content being viewed by others
Availability of Data and Material
All the data associated with this research has been presented in this paper.
References
Kaur A et al (2014) Nano molar detection of Al 3+ in aqueous medium and acidic soil using chromone based fluorescent organic nanoparticles (FONPs). Anal Methods 6(21):8752–8759
Maya S et al (2016) Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed Pharmacother 83:746–754
Capriello T et al (2019) Effects of aluminium and cadmium on hatching and swimming ability in developing zebrafish. Chemosphere 222:243–249
Frankowski M, Zioła-Frankowska A, Siepak J (2010) New method for speciation analysis of aluminium fluoride complexes by HPLC–FAAS hyphenated technique. Talanta 80(5):2120–2126
Ma Y-H et al (2010) A new aluminum (III)-selective potentiometric sensor based on N, N′-propanediamide bis (2-salicylideneimine) as a neutral carrier. Mater Sci Eng C 30(1):209–213
Xia D-H et al (2019) Detection of atmospheric corrosion of aluminum alloys by electrochemical probes: theoretical analysis and experimental tests. J Electrochem Soc 166(12):B1000
Bhogal S et al (2020) Core-shell structured molecularly imprinted materials for sensing applications. TrAC Trends Anal Chem 116043
Bhogal S et al (2019) Surface molecularly imprinted carbon dots based core-shell material for selective fluorescence sensing of ketoprofen. J Fluoresc 29(1):145–154
Malešev D, Kuntić V (2007) Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J Serb Chem Soc 72(10):921–939
Keri RS et al (2014) Chromones as a privileged scaffold in drug discovery: A review. Eur J Med Chem 78:340–374
Zhang H et al (2012) Spiralisones A-D: acylphloroglucinol hemiketals from an Australian marine brown alga. Zonaria Spiralis Organic & Biomolecular Chemistry 10(48):9671–9676
Raj T et al (2009) Mechanism of unusual formation of 3-(5-phenyl-3H-[1, 2, 4] dithiazol-3-yl) chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides and their antimicrobial evaluation. Eur J Med Chem 44(8):3209–3216
Medeiros P et al (2016) Raman microspectroscopy for probing the impact of a dietary antioxidant on human breast cancer cells. Food Funct 7(6):2800–2810
Maicheen C et al (2013) Synthesis, topoisomerase I inhibitory and cytotoxic activities of chromone derivatives. Med Chem 9(3):329–339
Legoabe LJ, Petzer A, Petzer JP (2012) Selected C7-substituted chromone derivatives as monoamine oxidase inhibitors. Bioorg Chem 45:1–11
Gül DŞ, Ogutcu H, Hayvalı Z (2020) Investigation of photophysical behaviours and antimicrobial activity of novel benzo-15-crown-5 substituted coumarin and chromone derivatives. J Mol Struct 1204:127569
Khanna R et al (2015) Absorption and fluorescent studies of 3-hydroxychromones. J Fluoresc 25(5):1159–1163
Rohman MA et al (2019) Specific solvent effect on the photophysical behavior of substituted chromones: A combined fluorescence. DFT and MD study Chemical Physics 517:67–79
Bhardwaj S, Maurya N, Singh AK (2018) Chromone based fluorescent organic nanoparticles for high-precision in-situ sensing of Cu2+ and CN− ions in 100% aqueous solutions. Sens Actuators B Chem 260:753–762
Gupta VK, Mergu N, Singh AK (2014) Fluorescent chemosensors for Zn2+ ions based on flavonol derivatives. Sens Actuators B Chem 202:674–682
Gharpure M et al (2012) Synthesis and biological evaluation of 3-hydroxy-2-phenyl-4H-chromen-4 ones. Int J Knowl Eng 3:148–150
Li C-R et al (2016) A chromone-derived Schiff-Base ligand as Al 3+ turn on fluorescent sensor: synthesis and spectroscopic properties. J Fluoresc 26(1):345–353
Jakubek M et al (2017) Water soluble chromone Schiff base derivatives as fluorescence receptor for aluminium (III). Supramol Chem 29(1):1–7
Fan L et al (2014) A chromone Schiff-base as Al (III) selective fluorescent and colorimetric chemosensor. J Lumin 155:84–88
Grazul M, Budzisz E (2009) Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord Chem Rev 253(21–22):2588–2598
Tian L et al (2019) A novel chromone derivative as dual probe for selective sensing of Al (III) by fluorescent and Cu (II) by colorimetric methods in aqueous solution. Journal of Photochemistry and Photobiology A: Chemistry 382:111955
Pang B-J, Li C-R, Yang Z-Y (2018) A novel chromone and rhodamine derivative as fluorescent probe for the detection of Zn (II) and Al (III) based on two different mechanisms. Spectrochim Acta Part A Mol Biomol Spectrosc 204:641–647
Acknowledgements
SB, PS, PR, and AKM are thankful to the UGC-SAP and the Chemistry Department, Punjabi University, Patiala, for providing lab and instrument facilities. KK is thankful to Mata Gujri College, Fatehgarh Sahib, Punjab, for providing lab facilities.
Funding
The authors did not receive support from any organization for the submitted work and have no financial or non- financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Shikha Bhogal: Conceptualization, Methodology, Data curation, Writing-original draft, Visualization, Validation. Promila Sharma: Conceptualization, Methodology, Data curation, Writing-original draft, Visualization, Validation. Pooja Rani: Conceptualization, Methodology, Data curation, Writing-original draft, Visualization, Validation. Kuldeep Kaur: Investigation, Methodology, Project Administration, Resources, Software, Supervision, Validation, Visualization, Writing-review and editing. Ashok Kumar Malik: Project administration, Investigation, Supervision, Writing-review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhogal, S., Sharma, P., Rani, P. et al. Synchronous Fluorescence Determination of Al3+ Using 3-Hydroxy-2-(4-Methoxy Phenyl)-4H-Chromen-4-One as a Fluorescent Probe. J Fluoresc 32, 359–367 (2022). https://doi.org/10.1007/s10895-021-02855-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02855-5