Abstract
The varied applications of nanotechnology have paved way for several breakthroughs in the realm of biomedical technology. In this challenging era when illness multiplies, timely and accurate disease diagnosis is very important. Thus, well founded novel approaches matter very much in areas like disease diagnosis and monitoring. Nanomedicine has tremendous implications in the given context. An elevated cholesterol concentration in blood is risky and is associated with cardiovascular diseases (CVD). CVD remains the No. 1 global cause of death and hence there is an urge to understand cholesterol level and take preventive measures. Highly fluorescent graphene quantum dots (GQs) are well known for their biocompatibility, non toxicity and aqueous solubility. Here in we report an easy and sensitive non enzymatic based cholesterol detection using digitonin conjugated graphene quantum dots (GDG). Selectivity studies and the cholesterol detection in human blood serum suggests the probe to be reliable and selective for blood cholesterol monitoring.
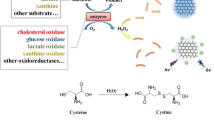
Graphical abstract
Digitonin conjugated fluorescent graphene quantumdots, an efficient probe for cholesterol sensing







Similar content being viewed by others
Data Availability
Not Applicable.
References
Schroeder KL, Goreham RV, Nann T (2016) Graphene quantum dots for Theranostics and bioimaging. Pharm Res 33:2337–2357
Miao P, Han K, Tang Y, Wang B, Lin T, Cheng W (2015) Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale 7:1586–1595
Hola K, Zhang Y, Wang Y, Giannelis EP, Zboril R, Rogach AL (2014) Carbon dots - emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 9:590–603
Kumawat MK, Thakur M, Gurung RB, Srivastava R (2017) Graphene quantum dots from Mangifera indica: application in near-infrared bioimaging and intracellular Nanothermometry. ACS Sustain Chem Eng 5:1382–1391
Hong G, Diao S, Antaris AL, Dai H (2015) Carbon Nanomaterials for biological imaging and Nanomedicinal therapy. Chem Rev 115:10816–10906
Thakur M, Mewada A, Pandey S, Bhori M, Singh K, Sharon M, Sharon M (2016) Milk-derived multi-fluorescent graphene quantum dot-based cancer theranostic system. Mater Sci Eng C Mater Biol Appl 67:468–477
Thakur M, Kumawat MK, Srivastava R (2017) Multifunctional graphene quantum dots for combined photothermal and photodynamic therapy coupled with cancer cell tracking applications. RSC Adv 7:5251–5261
Cheng F, An X, Zheng C, Cao S (2015) Green synthesis of fluorescent hydrophobic carbon quantum dots and their use for 2,4,6-trinitrophenol detection. RSC Adv 5:93360–93363
Sheng Qian Z, Yue Shan X, Jing Chai L, Rong Chen J, Feng H (2014) Dual-colored graphene quantum dots-labeled nanoprobes/graphene oxide: functional carbon materials for respective and simultaneous detection of DNA and thrombin. Nanotechnology 25:415501
Tang L, Ji R, Cao X, Lin J, Jiang H, Li X, Teng KS, Luk CM, Zeng S, Hao J, Lau SP (2012) Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 6:5102–5110
Dong Y (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon;v. 50:pp. 4738-43-2012 v.50 no.12
Wu X, Tian F, Wang W, Chen J, Wu M, Zhao JX (2013) Fabrication of highly fluorescent graphene quantum dots using l-glutamic acid for in vitro/in vivo imaging and sensing. J Mater Chem C 1:4676–4684
Liu R, Wu D, Feng X, Müllen K (2011) Bottom-up fabrication of Photoluminescent Graphene quantum dots with uniform morphology. J Am Chem Soc 133:15221–15223
Mewada A, Pandey S, Shinde S, Mishra N, Oza G, Thakur M, Sharon M, Sharon M (2013) Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater Sci Eng C Mater Biol Appl 33:2914–2917
Pandey S, Mewada A, Thakur M, Pillai S, Dharmatti R, Phadke C, Sharon M (2014) Synthesis of mesoporous silica oxide/C-dot complex (meso-SiO2/C-dots) using pyrolysed rice husk and its application in bioimaging. RSC Adv 4:1174–1179
Nishikawa M, Nojima S, Akiyama T, Sankawa U, Inoue K (1984) Interaction of digitonin and its analogs with membrane cholesterol. J Biochem 96:1231–1239
Edwards CH, Edwards GA, Gadsden EL (1964) Tomatine and Digitonin as precipitating agents in the estimation of cholesterol. Anal Chem 36:420–421
Batra N, Tomar M, Gupta V (2015) ZnO-CuO composite matrix based reagentless biosensor for detection of total cholesterol. Biosens Bioelectron 67:263–271
Nauck M, März W, Wieland H (2000) Is lipoprotein(a) cholesterol a significant Indicator of cardiovascular risk? Clin Chem 46:436–437
Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, Bjornsson E, Norddahl GL, Jonasdottir A, Jonasdottir A, Eggertsson HP, Gretarsdottir S, Thorleifsson G, Indridason OS, Palsson R, Jonasson F, Jonsdottir I, Eyjolfsson GI, Sigurdardottir O, Olafsson I, Danielsen R, Matthiasson SE, Kristmundsdottir S, Halldorsson BV, Hreidarsson AB, Valdimarsson EM, Gudnason T, Benediktsson R, Steinthorsdottir V, Thorsteinsdottir U, Holm H, Stefansson K (2019) Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol 74:2982–2994
Zhang M, Yuan R, Chai Y, Chen S, Zhong X, Zhong H, Wang C (2012) A cathodic electrogenerated chemiluminescence biosensor based on luminol and hemin-graphene nanosheets for cholesterol detection. RSC Adv 2:4639–4641
Lee Y-J, Park J-Y (2010) Nonenzymatic free-cholesterol detection via a modified highly sensitive macroporous gold electrode with platinum nanoparticles. Biosens & bioelectron 26:1353–1358
Li Y, Bai H, Liu Q, Bao J, Han M, Dai Z (2010) A nonenzymatic cholesterol sensor constructed by using porous tubular silver nanoparticles. Biosens & bioelectron 25:2356–2360
Raj V, Jaime R, Astruc D, Sreenivasan K (2011) Detection of cholesterol by digitonin conjugated gold nanoparticles. Biosens Bioelectron 27:197–200
Raj V, Vijayan AN, Joseph K (2015) Cysteine capped gold nanoparticles for naked eye detection of E. coli bacteria in UTI patients. Sens Bio-Sens Res 5:33–36
Sun L, Li S, Ding W, Yao Y, Yang X, Yao C (2017) Fluorescence detection of cholesterol using a nitrogen-doped graphene quantum dot/chromium picolinate complex-based sensor. J Mater Chem B 5:9006–9014
Shanti Krishna A, Radhakumary C, Sreenivasan K (2015) Detection and imaging of fatty plaques in blood vessels using functionalized carbon dots. Anal Methods 7:9482–9488
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 50:4738–4743
Krishna AS, Nair PA, Radhakumary C, Sreenivasan K (2016) Carbon dot based non enzymatic approach for the detection and estimation of glucose in blood serum. Mater Res Express 3:055001
Gopu CL, Shanti Krishna A, Sreenivasan K (2015) Fluorimetric detection of hypochlorite using albumin stabilized gold nanoclusters. Sensors Actuators B Chem 209:798–802
Ye Z, Tang R, Wu H, Wang B, Tan M, Yuan J (2014) Preparation of europium complex-conjugated carbon dots for ratiometric fluorescence detection of copper(ii) ions. New J Chem 38:5721–5726
Krishna AS, Radhakumary C, Sreenivasan K (2013) In vitro detection of calcium in bone by modified carbon dots. Analyst 138:7107–7111
Krishna AS, Radhakumary C, Antony M, Sreenivasan K (2014) Functionalized carbon dots enable simultaneous bone crack detection and drug deposition. J Mater Chem B 2:8626–8632
He YQ, Liu SP, Kong L, Liu ZF (2005) A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim Acta A Mol Biomol Spectrosc 61:2861–2866
Babic M, Horák D, Jendelová P, Glogarová K, Herynek V, Trchova M et al (2009) Poly(N,N-dimethylacrylamide)-coated maghemite nanoparticles for stem cell labeling. Bioconjug Chem 20:283–294
Acknowledgments
Authors are grateful to Dr. Francis Fernandez and Ms. Susan for TEM analysis and CSIR-- NIIST, Trivandrum for HRTEM images.
Code Availibility
Not Applicable.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to declare relevant to the content of this article.
Competing Interests
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 361 kb)
Rights and permissions
About this article
Cite this article
Ayilliath, S.K., Nair, S.R., Lakshmi, G.C. et al. Functionalised Graphene Quantum Dots for Cholesterol Detection in Human Blood Serum. J Fluoresc 31, 847–852 (2021). https://doi.org/10.1007/s10895-021-02712-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02712-5