Abstract
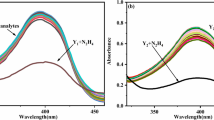
An efficient protocol for the synthesis of new rhodol derivatives has been developed. The synthesis involves condensation of 2-hydroxy benzophenone derivatives with 1, 3-dihydroxy benzene derivatives in solvents such as ionic liquid (N-methyl-2-pyrrolidonium hydrogen sulfate) and methane sulphonic acid. Both ionic liquid and methane sulphonic acid were found to be promising self-catalyzed solvents to bring out the conversion to form desired rhodols in excellent yields. In N-methyl-2-pyrrolidonium hydrogen sulfate reaction proceeds faster compared to methane sulphonic acid. The new fluorophores are investigated for their photophysical properties in various microenvironments and systematically characterized by means of density functional theory and time dependent density functional theory. Photophysical properties of the new rhodafluors found sensitive towards change in the pH of media and thus can be used as efficient pH sensors.













Similar content being viewed by others
References
Burdette SC, Lippard SJ (2002) The Rhodafluor family. An initial study of potential Ratiometric fluorescent sensors for Zn 2 +. Inorg Chem 41:6816–6823. doi:10.1021/ic026048q
Whitaker JE, Haugland RP, Ryan D, et al. (1992) Fluorescent rhodol derivatives: versatile, photostable labels and tracers. Anal Biochem 207:267–279. doi:10.1016/0003-2697(92)90011-U
Peng, T (2009) Rhodol fluorophores and fluorescent probes for the detection and imaging of reactive oxygen species. doi:10.5353/th_b4175792
Peng T, Yang D (2010) Construction of a library of rhodol fluorophores for developing new fluorescent probes. Org Lett 12:496–499. doi:10.1021/ol902706b
Sauers RR, Husain SN, Piechowski AP, Bird GR (1987) Shaping the absorption and fluorescence bands of a class of efficient, photoactive chromophores: synthesis and properties of some new 3H-xanthen-3. Dyes Pigments 8:35–53. doi:10.1016/0143-7208(87)85004-0
Yang BH, Buchwald SL (1999) Palladium-catalyzed amination of aryl halides and sulfonates. J Organomet Chem 576:125–146
Wolfe, JP, Wagaw, S, Buchwald, SL (1998) Rational development of practical catalysts for aromatic carbon - nitrogen bond formation. 31:805–818
Clark MA, Duffy K, Tibrewala J, Lippard SJ (2003) Synthesis and metal-binding properties of chelating fluorescein derivatives. Org Lett 5:2051–2054. doi:10.1021/ol0344570
Smith GA, Metcalfe JC, Clarke SD (1993) The design and properties of a series of calcium indicators which shift from rhodamine-like to fluorescein-like fluorescence on binding calcium. J Chem Soc Perkin Trans 2:1195. doi:10.1039/p29930001195
Lee LG, Berry GM, Chen CH (1989) Vita blue: a new 633-nm excitable fluorescent dye for cell analysis. Cytometry 10:151–164. doi:10.1002/cyto.990100206
Poronik YM, Clermont G, Blanchard-Desce M, Gryko DT (2013) Nonlinear optical chemosensor for sodium ion based on rhodol chromophore. J Org Chem 78:11721–11732. doi:10.1021/jo401653t
Yamada C, Sasaki K, Matsumura S, Toshima K (2007) Aryl C-glycosylation using an ionic liquid containing a protic acid. Tetrahedron Lett 48:4223–4227
Buzzeo MC, Evans RG, Compton RG (2004) Non-haloaluminate room-temperature ionic liquids in electrochemistry--a review. Chemphyschem 5:1106–1120. doi:10.1002/cphc.200301017
Brouwer AM (2011) Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report. Pure Appl Chem 83:2213–2228. doi:10.1351/PAC-REP-10-09-31
Thorat KG, Ray AK, Sekar N (2016) Modulating TICT to ICT characteristics of acid switchable red emitting boradiazaindacene chromophores: Perspectives from synthesis, photophysical, hyperpolarizability and TD-DFT studies. Dye Pigment. doi:10.1016/j.dyepig.2016.08.049
Chaudhari AS, Parab YS, Patil V, et al. (2012) Intrinsic catalytic activity of Bronsted acid ionic liquids for the synthesis of triphenylmethane and phthalein under microwave irradiation. RSC Adv 2:12112. doi:10.1039/c2ra21803h
Liu Q-H, Liu J, Guo J-C, et al. (2009) Preparation of polystyrene fluorescent microspheres based on some fluorescent labels. J Mater Chem 19:2018. doi:10.1039/b816963b
Thorat KG, Kamble PS, Ray AK, Sekar N (2015) Novel Pyrromethene dyes with N-ethyl carbazole at meso position: comprehensive photophysical, lasing, photostability and TD-DFT study. Phys Chem Chem Phys 17:17221–17236. doi:10.1039/C5CP01741F
Frisch, MJ, Trucks, GW, Schlegel, HB, et al. (2009) Gaussian 09 C.01. Gaussian Inc., Wallingford CT
Treutler O, Ahlrichs R (1995) Efficient molecular numerical integration schemes. J Chem Phys 102:346. doi:10.1063/1.469408
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372. doi:10.1063/1.464304
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Hehre WJ (1976) Ab initio molecular orbital theory. Acc Chem Res 9:399–406. doi:10.1021/ar50107a003
Gabe Y, Ueno T, Urano Y, et al. (2006) Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: improvement of highly sensitive fluorescence probe for nitric oxide. Anal Bioanal Chem 386:621–626. doi:10.1007/s00216-006-0587-y
Furche F, Ahlrichs R (2002) Adiabatic time-dependent density functional methods for excited state properties. J Chem Phys 117:7433. doi:10.1063/1.1508368
Scalmani G, Frisch MJ, Mennucci B, et al. (2006) Geometries and properties of excited states in the gas phase and in solution: theory and application of a time-dependent density functional theory polarizable continuum model. J Chem Phys 124:94107. doi:10.1063/1.2173258
Thorat KG, Bhakhoa H, Ramasami P, Sekar N (2015) NIR-emitting Boradiazaindacene fluorophores -TD-DFT studies on electronic structure and Photophysical properties. J Fluoresc 25:69–78. doi:10.1007/s10895-014-1481-1
Thorat KG, Kamble P, Mallah R, et al. (2015) Congeners of Pyrromethene-567 dye: perspectives from synthesis, photophysics, photostability, laser and TD-DFT theory. J Org Chem 80:6152–6164. doi:10.1021/acs.joc.5b00654
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335. doi:10.1016/0009-2614(96)00349-1
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093. doi:10.1021/cr9904009
Bauernschmitt R, Ahlrichs R (1996) Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem Phys Lett 256:454–464. doi:10.1016/0009-2614(96)00440-X
Acknowledgments
One of the authors, KGT, is thankful to the Council of Scientific and Insdustrial Research (CSIR), India for Junior and Senior Research Fellowships, and RM is thankful to University Grant Comission for Junior and Senior Research Fellowships under SAP programme. SP is thankful to TEQIP (Technical Education Quality Improvement Program) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supriya S. Patil and Kishor G. Thorat contributed equally equally to this work.
Electronic supplementary material
[Supporting Information contains optimized geometry parameter table, absorption emission spectra, 1H, 13C and LCMS spectra, etc.]
ESM 1
(DOCX 1155 kb)
Rights and permissions
About this article
Cite this article
Patil, S.S., Thorat, K.G., Mallah, R. et al. Novel Rhodafluors: Synthesis, Photophysical, pH and TD-DFT Studies. J Fluoresc 26, 2187–2197 (2016). https://doi.org/10.1007/s10895-016-1915-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1915-z