Abstract
The chemiluminescence (CL) of oxidation of non-steroidal anti-inflammatory drugs (NSAIDs) by Ce(IV) ions, was recorded in the presence and absence europium(III) ions, in solution of pH ~ 4 of solution. Kinetic curves and CL emission spectra of the all studied systems were discussed. CL of measurable intensity was observed in the Ce(IV)–NP–Eu(III) reaction system only in acidic solutions. The CL spectrum rcegistered for this system shows emission bands, typical of Eu(III) ions, with maximum at λ ~ 600 nm. The chemiluminescent method, based on Eu(III) emission in reaction system of NP-Ce(IV)–Eu(III) in acid solution was therefore used for the determination of naproxen in mixture of non-steroidal anti-inflammatory drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naproxen(NP), ibuprofen(IB) and indomethacin (IM) are non-steroidal anti-inflammatory drugs (NSAID). Chemically, NP and IB belong to the 2-arylpropionic acid family, whereas IM belongs to the class of arylacetic acid. The NSAID as a group are diverse in chemical structure but are consistent in their ability to inhibit the enzyme cyclooxygenase, regarded as a marker for inflammation [1]. The complexation processes of naproxen(NP) or ibuprofen(IB) or indomethacin (IM) with lanthanide ions depend of pH values. In acidic solution, the degree of complexation is small [2]. The trivalent lanthanide ions, especially Eu(III) and Tb(III), display spectroscopic properties of high photoluminescence efficiencies, long excited-state lifetimes band narrow emission spectra, all of which make them be widely used as analytical luminescent probes for a variety of chemical and biological applications [3–5].
Chemiluminescence can be achieved by direct reactions or by energy-transfer mechanisms. The CL phenomenon can be applied as detection technique for the monitoring of a wide variety of compounds in diverse fields, such as biomedical, environmental and can be considered as an alternative to other detection modes such as: UV/Vis spectroscopy, fluorescence [6]. In this work, the use of sensitized chemiluminescence of lanthanide ions for determination of naproxen in mixture of NSAIDs is studied. It was observed that only the CL signal of the Ce(IV)- Naproxen system was significantly increased in the presence of Eu(III) ions.
Experimental
Ultra weak photon emission was recorded with the use of the earlier described equipment [7]. The fluorescence spectra were recorded using F-7000 HITACHI spectrofluorimeter.
Chemicals
All chemicals used were of analytical reagent grade and doubly distilled water was used throughout the experiments. Naproxen sodium and indomethacin were from Sigma, ibuprofen sodium was from Fluka.
Their stock standard solutions (2 × 10−3 molL−1) were prepared by dissolving appropriate amount of naproxen sodium and ibuprofen sodium in water and diluting to 100 ml with water. A stock standard solution of indomethacin (6 × 10−4 molL−1) was prepared by dissolving an appropriate amount of IM with 0.1 molL−1 hydrochloric acid and then diluting to 100 mL with water. Working standard solutions of naproxen sodium were prepared of diluted the stock solution with water.
Optimization of Experimental Conditions
The optimum conditions for the naproxen determinations were carried out using the 1 × 10−6 molL−1 concentration of NP. The effect of Ce(IV) concentration on the CL reaction was examined in the range of 5.0 × 10−4 to 4.0 × 10−3 molL−1 in 0.015, 0.025, 0.05, 0.15 or 0.3 molL−1 H2SO4.The most integrated CL light sum was obtained when the concentration of Ce(IV) was 2.0 × 10−3 mol L−1in 0.015 molL−1 H2SO4. For the NP-Ce(IV)-Eu(III) system, the optimum concentration of europium(III) ions, using the following range of concentrations 1.0 × 10−4 - 1.0 × 10−2 molL−1 was determined. In the case of concentration of Eu(III) ions above 1.0 × 10−3 molL−1 the CL light intensity sums did not change, so as the optimal concentration of Eu(III) ions for the determination of NP 2.0 × 10−3 mol L−1 was chosen.
Measurement Procedure
All of the chemiluminescence studies were performed in the same manner. Solution of cerium(IV) ions was added to solutions containing NSAID (1 mL solutions of appropriate concentrations) or NSAID and Eu(III) ions. The experiments were conducted in acidic solutions (pH ~ 4). The total volume was 4 mL. The determination of naproxen in water, pharmaceutical preparations was carried out measuring the CL light intensity sums of the reaction systems applying the cut-off filters method, with the use of a filter transmitting emission of λ > 585 nm, which was placed between the photomultiplier and the measurement cell. CL light intensity sums were calculated as the area under these curves [4].
Sample Preparation
Tablets
Not less than 10 tablets were weighed and finely powdered. Specific amounts of analyte were weighed accurately, dissolved in water in a small beaker. The solution was filtered, and the residue was washed with water several times. Then it was transferred into a 250 mL calibrated flask and diluted to the specified volume with water. Working solutions were prepared by appropriate dilution of this sample solution so that the final analytic concentrations were within the working range.
Ointment
Accurately weighed out amount of the ointment was dissolved in water (40 °C) and 1 mL 0.1 molL−1 NaOH while vigorously mixing. Next the solution was cooled in a refrigerator, in order to separate from vaseline, and transferred into a 100 ml calibrated flask and diluted to volume with water. The final concentration of NP was within the working range.
Urine
Urine samples were obtained from two healthy volunteers. Known amounts of standard solutions of naproxen were introduced into 1 mL of fresh urine samples. The typical naproxen urinary concentration was 0.2–20 μg/ml [8]. This urine sample was mixed with a 10 mL of 0.1 molL−1 Ba(OH)2 solution and a 9 mL of 0.1 molL−1 ZnSO4 solution in order to remove protein and reducing substances [9, 10]. The resulting solution was diluted to 50 mL with doubly distilled water and centrifuged at 3000 for 15 min. The supernatant solution was stored and maintained below 5 °C. For analysis 1 ml of solution was used.
Results and Discussion
Effect of Presence of the Europium(III) Ions on the CL Intensity of the Ce(IV)-NSAID Systems
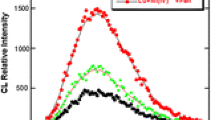
The chemiluminescence of systems containing NP or IB or IM and Ce(IV) ions as oxidizer in solution at pH ~ 4 in the absence and in the presence europium(III) ions were studied. Chemiluminescence in the system NSAID-Ce(IV) was obtained, when the concentration of NSAID was higher than 2 × 10−7 molL−1 for NP and 4 × 10−3 molL−1 for IB and 2 × 10−4 molL−1 for IM. For all systems the course of the kinetic curves of CL were similar, with maximum after initiation of the reaction by introducing Ce(IV) ions. The emission at the level of the background was obtained in about 30 s after the reaction initiation. Introduction of Eu(III) ions into the system NSAID-Ce(IV) resulted in a strong increase in the emission intensity only in the system containing naproxen without a change in the kinetic curve character (curves 3and 4 in Fig. 1). The intensity of CL of the systems IB-Ce(IV) and IB-Ce(IV)-Eu(III) or IM-Ce(IV) and IM-Ce(IV)-Eu(III) were the same. The kinetics of the chemiluminescence decay of NP-Ce(IV) and NP-Ce(IV) – Eu(III) were monoexponential.
The kinetic curves of CL decay in the NP-Ce(IV) system versus the concentration of NP: 6 × 10−7 molL−1 (curve 1); 2 × 10−6 molL−1 (curve 2) and NP-Ce(IV)-Eu(III) system versus the concentration of NP: 6 × 10−7 molL−1 (curve 3); 2 × 10−6 molL−1 (curve 4). The concentration of Eu(III) ions was 2 × 10−3 molL−1; the initial concentration of Ce(IV) was 2 × 10−3 molL−1, pH ~ 4
Spectral analysis of the chemiluminescence in the systems studied was performed using the method of cut-off filters as described earlier [11]. The spectra show a broad emission band in the range 400–600 nm, with a maximum at λ ~ 450 nm for NP and 480 nm for IM or 420 nm for IB, which is typical of the CL systems with the compounds containing excited carbonyl groups as emitters [12]. The spectral distribution of CL of NP-Ce(IV)-Eu(III) system dependent on the concentration of europium(III) ions. The spectral distribution, observed for the molar ratio Eu: NP = 10: 1, shows a broad band at 450 nm and a second band centered at λ = 600 nm (spectrum 2 in Fig. 2) corresponding to the 5D0 → 7 F1 and 5D0 → 7 F2 transition of the Eu(III) ion [13]. For higher molar ratio (Eu: NP above 100: 1) the dominant band is band typical for Eu(III) with a maximum at λ ~ 600 nm (spectrum 4 in Fig. 2). This means that the presence of the lanthanide ion is responsible for the increase in the CL intensity of NP-Ce(IV)-Eu(III) system.
The normalized spectral distribution of CL of the systems: NP-Ce(IV) (spectrum 1) and NP-Ce(IV)-Eu(III) versus the molar ratio Eu(III): NP = 10: 1 (spectrum 2); 40: 1 (spectrum 3) and 100: 1 (spectrum 4). The concentration of NP was 1 × 10−5 molL−1; the initial concentration of Ce(IV) was 2 × 10−3 molL−1, pH ~ 4
In order to identify the role of the lanthanide ion in the reaction mixture NP-Ce(IV)-Eu(III), the process of Ln(III) complexation with NP in the pH range about 4–5 was studied. In the investigated pH region for all of the molar ratios used (Eu: NP = 10:1; 40:1 and 100:1) the luminescence of Eu(III) was not observed, applying λex typical for NP, i.e.: 270 nm, 317 nm and 327 nm. The recorded luminescence spectra show only one large unstructured band centered at around 353 nm (spectra 1, 2, 3 in Fig. 3), typical for this drug [14]. Using λex = 394 nm (typical for Eu(III) ions) the same luminescence spectrum (spectrum 4 in Fig. 3) as in the case of EuCl3 solution was obtained, which is typical for uncomplexed Eu(III) ions [11]. This indicates that the complex formation did not occur place in the chemiluminescent reaction system NP-Ce(IV)-Eu(III).
As follows from Fig. 1 the presence of Eu(III) ions have no effect on the rate of the naproxen oxidation with cerium(IV) ions. In the chemiluminescent experimental conditions europium(III) ions are uncomplexed and because of that a ligand to metal transfer process cannot appear (Fig. 3). The presence of europium(III) bands in the CL spectrum of the NP-Ce(IV)-Eu(III) system indicates that the excitation of Eu(III) ions in the reaction mixture is a result of the energy transfer process from the excited product of NP oxidation to the uncomplexed Eu(III) ions.
Oxidative decarboxylation of substituted phenylacetic acids can be mediated by metallic and nonmetallic oxidants or by electrolytic means [15]. When naproxen was treated with cerium (IV) or peroxodisulfate, the product pattern was similar to that from photolysis [16]. The major photoproducts obtained by irradiation of the drug under aerobic conditions are 6-methoxy-2-(1-hydroxyethyl)-naphthalene and 6-methoxy-2-acetyl-naphthalene, and a small amount of 6-methoxy-2-ethyl-naphthalene. The 6-methoxy-2-acetyl-naphthalene shows the emission band centered at around 440 nm [17], which correspond to the chemiluminescent spectrum.
Analytical Application
The reaction mixture of NP-Ce(IV)-Eu(III) was used for the determination of the naproxen concentration, with applying the cut-off filters method. A linear dependence of the CL light intensity sums of the reaction system on the NP concentration, with the use of a filter transmitting emission of λ > 585 nm, was obtained in the NP concentration range of 4 × 10−8 molL−1 (9 ng/mL) to 2 × 10−6 molL−1(460 ng/mL) (correlation cofficient r = 0.9998) with the detection limit of 1.1 × 10−8 molL−1 (2.5 ng/mL). It is six times lower than determination in reaction system without europium(III) ions [18].
Interference
The effect of some common inorganic ion and excipient species on the determination of 2.0 × 10−7 molL−1 naproxen solution was studied. The tolerance limit was taken as the recovery in the range 95%–105%. The tolerance ratios were as follows: 5000-fold for indomethacin, ibuprofen, K(I), Na(I), Mg(II), Ca(II); Zn(II); NO −3 , SO4 2−; 500-fold for CO3 2−; 100-fold for Fe(III) starch, glycine, glucose; and 30-fold for Fe(II). These tolerance ratios were higher than in system without europium(III) ions [18].
Applications
The proposed method was applied to the determination of Naproxen in commercially available pharmaceuticals. The obtained results are shown in Table 1. As can be seen, there are no significant differences between the labeled values and those obtained by the suggested method. A mean recovery values were in the range 98–104%.
The accuracy studies for the determination of naproxen in human urine were conducted with three known concentrations of the compound. Results of recovery studies for urine are given in Table 2. Each value represents the mean of five measurements.
Conclusion
The reaction system NSAID-Ce(IV) belongs to the group systems generating ultra-weak emission characteristic for excited carbonyl species formed as a result of NSAID oxidation. The presence of Eu(III) ions into the systems of IB-Ce(IV) and IM-Ce(IV) did not influence on CL intensity, however the Eu(III) ions are effective activators in the NP-Ce(IV) system. The CL reaction system of NP-Ce(IV)-Eu(III), as these studies have shown, can be analytically useful. This method gives a lower detection limit and a large tolerance of on the presence of inorganic ion and excipient species compared with the NP-Ce(IV) system. The proposed chemiluminescent method, based on emission of the Eu(III) ions in the NP-Ce(IV)-Eu(III) system, is simple, accurate and precise and allows the determination of naproxen in pharmaceutical preparations and in human urine.
References
Vane JR, Botting RM (1995) New insights into the mode of action of anti-inflammatory drugs. Inftamm Res 44(1):1–10
Telyuk OI, Beltyukova SV, Yegorova AV, Yagodkin BN (2007) Complex compounds of terbium(III) with some are non-steroidal anti-inflammatory drugs and their analytical applications. J Anal Chem 62(4):330–335
Li D, Du J, Lu J (2008) Europium(III)-senstized chemiluminescence determination of ibuprofen in pharmaceutical preparations and biological fluids. Anal Letters 41(8):1366–1374
Kaczmarek M, Lis S (2009) Chemiluminescence determination of tetracyclines using Fenton system in the presence europium(III) ions. Anal Chim Acta 639(1–2):96–100
Al-Kindy SMZ, Suliman FEO (2007) Determination of ibuprofen in pharmacutical formulations using time-resolved terbium-sensitizied luminescence. Luminescence 22(4):294–301
Gámiz-Gracia L, García-Campaña AM, Huertas-Pérez JF, Lara FJ (2009) Chemiluminescence detection in liquid chromatography: Applications to clinical, pharmaceutical, environmental and food analysis—A review. Anal Chim Acta 640(1–2):7–28
Kaczmarek M, Lis S (2006) Luminescence characterization of the reaction system Histidin – KBrO3 – Tb(III) – H2SO4. J Fluorescence 16(6):825–830
Aresta A, Palmisano F, Zambonin CG (2005) Determination of naproxen in human urine by solid-phase microextraction coupled to liquid chromatography. J Pharm Biomed Anal 39(3–4):643–647
Malavolti NL, Pilosol D, Nieman TA (1985) Determination of cholesterol with a microporous membrane chemiluminescence cell with cholesterol oxidase in solution. Anal Chim Acta 170:199–207
Wang X, Zhao H, Nie L, Jin L, Zhang Z (2001) Europium sensitized chemiluminescence determination of rufloxacin. Anal Chim Acta 445(2):169–175
Staninski K, Kaczmarek M, Lis S, KomarD SA (2009) Spectral analysis in ultraweak emissions of chemi- and Electrochemluminescence systems. J Rare Earths 27(4):593–597
Ostakhov SS, Sharipov GL, Voloshin AI, Kazakov VP, Tolstikov GA (1986) Catalysis and sensitization of chemiluminescence at thermal decomposition of adamantylidene-adamantane-1,2-dioxetane by Tb, Eu and Ru compounds. Dokl Akad Nauk SSSR 287:1165–1168
Lis S (2002) Luminescence studies of lanthanide(III) ions in solution. J Alloys Comp 341(1–2):45–50
Velázquez MM, Valero M, Rodrignez LJ, Costa SMB, Santos MA (1995) Hydrogen binding in a non-steroidal anti-inflammatory drug, Naproxen. J Photochem Photobiol B Biol 29(1):23–31
Tanner DD, Osman SAA (1987) Oxidative decarboxylation. on the mechanism of the potassium persulfate promoted decarboxylation reaction. J Org Chem 52(21):4689–4693
Bosca F, Martinez-Manez R, Miranda MA, Primo J, Soto J, Vano L (1992) Oxidative decarboxylation of naproxen. J Pharm Sci 81(5):479–482
Valero M, Carrillo C (2004) Effect of binary and ternary polyethyleneglycol and/or β-cyclodextrin complexes on the photochemical and photosensitizing properties of naproxen. J Photochem Photobiol B Biol 74(2–3):151–160
Campiglio A (1998) Determination of naproxen with chemiluminescence detection. Analyst 123(7):1571–1574
Acknowledgments
Financial support from the Polish Ministry of Science and Higher Education; Grant NN204 028236 is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kaczmarek, M. Chemiluminescence of the Reaction System Ce(IV) - Non-Steroidal Anti-Inflammatory Drugs Containing Europium(III) Ions and its Application to the Determination of Naproxen in Pharmaceutical Preparations and Urine. J Fluoresc 21, 2201–2205 (2011). https://doi.org/10.1007/s10895-011-0923-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0923-2