Abstract
Predatory long-legged flies of the genus Medetera are important, but currently understudied, natural enemies of Scolytinae bark beetles such as Ips typographus. Medetera flies lay eggs on beetle-infested trees, where the developing larvae find their prey, but the chemical cues used by Medetera to locate infested trees are currently unknown. To identify odors attracting Medetera signaticornis, a species in Europe, headspace samples were collected at several time-points through different stages of I. typographus attacks on logs of Norway spruce (Picea abies). The headspace samples were analyzed using combined gas chromatography and mass spectrometry (GC–MS), and gas chromatography coupled with electroantennographic detection (GC-EAD) to determine compounds that stimulate M. signaticornis antennae. Antennae of M. signaticornis males and females were found to detect (–)-cis-verbenol, ( +)-trans-verbenol and myrtenol, which are known to be produced by bark beetles. Antennal responses were also observed for verbenene, isoterpinolene, α-pinene oxide, camphor, pinocamphone, terpinene-4-ol, myrtenal, borneol, α-terpineol, geranyl acetone, and verbenone, which are primarily produced by microorganisms, and α-pinene, α-fenchene, β-pinene, camphene, 3-carene, limonene, γ-terpinene, and terpinolene, known spruce tree compounds. In field experiments testing two synthetic blends containing 18 antennal active and two additional compounds 2-methyl-3-buten-2-ol and ipsdienol we observed significant attraction of M. signaticornis within 24 h. These attractive blends can form the basis for development of Medetera monitoring lures for use in future forest and pest management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Norway spruce (Picea abies (L.) Karst.), an ecologically and economically important conifer species, is increasingly being threatened by fungal disease (e.g., root and butt rot disease caused by Heterobasidion spp.) (Gunulf et al. 2013; Gomez-Gallego et al. 2022) and insect pests (Hannerz et al. 2002; Romashkin et al. 2020; Hlásny et al. 2021). One of the most severe pests on Norway spruce is the Eurasian eight-spined spruce bark beetle (Ips typographus Linnaeus) (Coleoptera: Curculionidae, Scolytinae) (Grégoire et al. 2015). Within the coniferous forest ecosystem, I. typographus feeds on dead or dying spruce trees and contributes to recycling of nutrients (Edmonds and Eglitis 1989). However, weather conditions such as strong wind, warm temperature and low rainfall can cause mass development of I. typographus in windthrown or draught-stressed spruce trees, and in consequence of a high beetle abundance even healthy trees get attacked (Rouault et al. 2006; Kärvemo and Schroeder 2010; Stadelmann et al. 2014). When colonizing living spruce trees, I. typographus uses an aggregation pheromone that facilitates mass attack (Birgersson et al. 1984; Schlyter et al. 1987) and introduces a blue-staining symbiotic fungus that metabolizes tree defense compounds toxic to the beetle (Hammerbacher et al. 2013; Wadke et al. 2016; Zhao et al. 2019). In addition, I. typographus performs better in warm temperatures, and physiological models predict more frequent outbreaks in European Picea forests due to climate change (Marini et al. 2017; Bentz et al. 2019). There is therefore a pressing need to find efficient and sustainable methods to control bark beetles.
Understanding chemo-ecological aspects underlying host finding and infestation of trees by the beetles as well as subsequent trophic interactions might facilitate the development of pest management methods by use of semiochemicals or natural enemies. Tree volatiles serve as signals in host recognition of bark beetles (Seybold et al. 2006). In coniferous trees, terpenoid compounds (primarily volatile mono- and sesquiterpenes or less-volatile diterpenes) are characteristic constitutive or inducible defense chemicals, respectively (Keeling and Bohlmann 2006; Martin et al. 2003). The emission of these compounds can increase as response to biotic and abiotic stress such as the attack by wood boring insects, high temperature or mechanical damage (Ghimire et al. 2016; Juráň et al. 2017; Holopainen et al. 2018).
Several natural enemies of bark beetle, such as parasitoids and predators, are attracted to bark beetle-infested trees by volatile chemical cues that originate from the host tree (e.g., mono- and sesquiterpenes), from bark beetles themselves (e.g., I. typographus produced oxygenated hemi- and monoterpene alcohols), and/or from associated microorganisms which the beetles transfer to the host trees and their offspring developing inside the galleries (e.g., oxygenated monoterpenes) (Leufvén et al. 1984; Leufvén and Birgersson 1987; Pettersson 2000; Kandasamy et al. 2016, 2021). Natural enemies can significantly reduce bark beetle populations and are considered to be environmentally safe and sustainable control agents (Wermelinger 2002; Kenis et al. 2007; Wegensteiner et al. 2015).
An important group of predator species of bark beetles are flies of the genus Medetera (Diptera: Dolichopodidae). At present these are not actively applied or considered as biocontrol agents in forest management. Medetera adults feed on a wide array of small invertebrates (Ulrich 2004), but the larvae of most tree trunk-dwelling Medetera species depend on bark beetles for their development. The adult long-legged flies can be observed on tree trunks from early spring throughout the whole summer and up to the first frost. Medetera females have been observed to oviposit in newly infested trunks shortly after infestation by bark beetles (Nicolai 1995; Wermelinger 2004). On infested trees, females inspect the bark surface with their ovipositor and lay their eggs near the entrance of bark beetle galleries. A few days later, the newly emerged larvae migrate into the galleries and start feeding on beetle eggs and larvae, and on pupae or newly emerged, callow bark beetle adults that are still concealed in the galleries and pupal chambers (Beaver 1966; Bickel 1985). To locate bark beetle-infested trees, the adult flies use volatile chemical cues (Hulcr et al. 2005, 2006). Information on the specific compounds required for host detection is scarce, but it is known that some Medetera spp., such as M. setiventris Thuneberg and M. melancholica Lundbeck, are attracted to the I. typographus aggregation pheromone, which consists of a mixture of (-)-cis-verbenol and 2-methyl-3-buten-2-ol (Hulcr et al. 2005, 2006). According to Hulcr et al. (2006), the number of M. setiventris attracted to traps increases when I. typographus aggregation pheromone is combined with ipsdienol. Furthermore, M. signaticornis Loew has been shown to be attracted to a mixture of host tree compounds such as α-pinene, β-pinene, camphene, and limonene dissolved in ethanol (Rudinsky et al. 1971), while α-pinene has been shown to stimulate oviposition in females of M. aldrichii Wheeler and seems to guide newly emerged larvae to prey gallery entrances (Fitzgerald and Nagel 1972).
Medetera signaticornis is described as one of the most common I. typographus predators in Europe (Ounap 2001; Wermelinger 2002), which makes it a good candidate species for use in a future biocontrol strategy. In this study, we tested the hypothesis that M. signaticornis adult flies use multiple semiochemicals to detect bark beetle-infested Norway spruce trees. In order to identify key compounds that attract M. signaticornis to infested spruce, we: i) compared the volatilome of bark beetle-infested standing trees, bark beetle-infested cut trees and non-infested spruce trees over time; ii) identified odor compounds from infested trees eliciting electroantennographic responses on the antennae of M. signaticornis adults; and iii) tested the effectiveness of synthetic olfactory-active compound blends under field conditions.
Material and Methods
Insects
Males and females of M. signaticornis were collected with a mouth aspirator from bark beetle-infested spruce trees (Picea abies) at two different sites (1 and 2, 57.150°N, 14.765°E and 57.127°N, 14.780°E, respectively) close to the SLU field research station in Asa, Småland province, Sweden, between May and August during 2018 and 2019. The average of the daily maximum temperature between May and August 2018 and 2019 at Asa was 24.3 ± 4.5 °C and 20.6 ± 3.5 °C, respectively, while the average daily precipitation during the same period was 1.65 ± 4.6 mm and 3.55 ± 8.1 mm (more detailed weather data can be accessed from the Asa weather station, Anon (2023)). Collected flies were placed individually in glass vials with humidified filter paper and transported to the laboratory, where they were kept starved at 4–8 °C until electrophysiology studies, which were carried out within a week collection of the flies.
Volatile Collection
Odor samples for chemical and electrophysiology analysis were collected from non-infested standing trees, infested standing trees, and infested cut trees at the two sites during 2018. A total of 11 healthy mature standing spruce trees, approximately 40–60 years age, were randomly selected at the two sites. Four of these trees were cut with a chainsaw (three at site 1 and one at site 2), and the fallen trunks and two standing trees at each site were baited with synthetic I. typographus pheromones (see below) to induce controlled I. typographus attacks. The remaining three trees (one from site 1 and two from site 2) were left without pheromone bait and used as controls.
The bait used to attract bark beetles to both cut and standing trees consisted of single dispensers containing the synthetic I. typographus aggregation pheromone (Pheroprax®, BASF, Limburgerhof, Germany). The dispensers were suspended at 3 m height (measured from the bottom of the tree) on the bark of the standing trees, and cut trees. Once I. typographus beetles had started excavating galleries in the baited trees, the dispensers containing synthetic bark beetle pheromones were removed and headspace collection was started.
A curved aluminum grid (area 32 cm × 32 cm, with 1.5 cm distance between grid and bark) was attached to each tree at specific collection points between 1.5 and 2 m tree height (measured from the bottom of the tree), to provide an open space for volatile release (Fig. 1A-C). For the eight infested cut or standing trees, the aluminum grid was affixed to cover the entrance hole(s) of one or two bark beetle galleries. To collect the volatiles emanating from the bark surface, the aluminum grid was covered with a polyester roasting bag (Toppits®, Cofresco Frischhalteprodukte GmbH, Minden, Germany) wrapped around the tree bark, giving an open bark surface area for volatile release of ~ 9 dm2. Nylon wire was used to tie the upper and lower edges of the polyester bag to the tree (Fig. 1B). The released volatiles were collected through an adsorbent column (3 × 55 mm PTFE Teflon® tube, inner diameter 3.0 mm, outer diameter 4.0 mm, filled with ~ 30 mg of Porapak Q (mesh 50/80, Waters, Milford, MA, USA)) that had been placed in the open space under the grid before wrapping with the polyester bag. The absorbent column was connected by silicone tubing to a battery-driven membrane pump (KNF NMP830KNDC, KNF, Sursee, Switzerland) and the air drawn through each column was adjusted to a flow rate of 150 mL.min−1 for 3 h. An additional Porapak Q column was connected to the pump for sampling potential contamination from the air outside the enclosed aluminum grid. Compared to the samples from the bark inside the grid, amounts of compounds trapped outside the grid were neglectable (data not shown). After headspace collection, the polyester bag was cut open and the column was transferred to a clean glass vial. All vials were sealed and transported to the laboratory in a container with ice. In the laboratory, each column was eluted with 500 µL of pentane (puriss p.a, Sigma-Aldrich, Saint Louis, MO, USA) and the eluate was stored at -20 °C. Headspace collections from the same collection points on the same experimental trees (C1-C7) were made approximately every 10–15 days over a period of two months (from 10th May for site 1 or 25th May for site 2 up to 25th July), until the new generation of bark beetles began to emerge. The starting time of headspace collections between sites differed slightly because bark beetles were active earlier at site 1 compared to site 2. However, collections C1 to C6 from sites 1 and 2 correspond to similar stages of bark beetle attack. Details about collection dates and climatic conditions can be found in the Supplementary Table 1. In total, we were able to collect and analyze 12 samples from non-infested Norway spruce trees, 26 samples from infested standing trees, and 25 samples from infested cut trees.
Setup used for headspace collections from logs of Norway spruce trees (Picea abies) through the different stages of Ips typographus attack. A) three sampled standing trees in one of the field sites; B) metal grid covered with a polyester roasting bag that was wrapped around the tree bark forming an enclosure for odor collections; C) material used: 1. battery; 2. sucking air pump; 3. adsorbent columns (3 × 55 mm); 4. air splitter; 5. Silicone tubing; 6. Cables to connect the battery to the air pump; 7. aluminum grid (area 32 × 32 cm); 8. polyester roasting bag
Analysis by Gas Chromatography Coupled to Mass Spectrometry (GC–MS)
All odor samples were concentrated to 100–150 µL and combined with 10 µL of heptyl acetate (100 ng µL−1) as internal standard. Then 2 µL of each sample were injected by auto-injector (G4567A) into a gas chromatograph (7890B, GC) with mass spectrometry detection (5977A MS) (all Agilent Technologies, Santa Clara, CA, USA). The GC was equipped with a 60 m × 0.25 mm fused silica column coated with DB-Wax (polyethylene glycol, df = 0.25 µm, Agilent Technologies). Helium was used as the mobile phase, with a constant flow rate of 35 cm.s−1. The temperature program increased from 40 °C (3 min hold) at 8 °C.min−1 to 225 °C, which was held for 10 min. Electron impact mass spectra were obtained at 70 eV. All compounds were tentatively identified by comparison of the mass spectra obtained against: i) reference mass spectra from our custom library (based on spectra of standards analyzed on our GC–MS devices), supplemented with commercially available MS libraries (NIST, Wiley), and ii) Kováts retention index (RI) with reference to public RI libraries (PheroBase) (El-Sayed 2016).
Insect Preparation and Analysis by Gas Chromatography Coupled to Electroantennographic Detection (GC-EAD)
To identify odor compounds from infested trees eliciting electroantennographic responses on antennae of M. signaticornis adults, the flies were gently inserted into a disposable plastic pipette tip with the narrow opening cut wider to let the fly’s head pass through while retaining the thorax and abdomen inside the tip. A piece of glass wool was stuffed into the tip behind the insect body to immobilize the fly. Two glass electrodes were filled with Beadle-Ephrussi Ringer solution and one was inserted into one of the fly’s eyes (indifferent electrode), while the other electrode was connected to a fly’s antenna and mounted on a 10 × preamplifier probe (Ockenfels Syntech GmbH, Buchenbach, Germany) attached to an Intelligent Data Acquisition Controller (IDAC-2, Ockenfels Syntech).
For each GC-EAD analysis, 2 µL of odor sample with internal standard were used (five replicates per fly sex). The GC column and temperature program applied were similar to those used for GC–MS analysis. Hydrogen was used as a mobile phase, at 45 cm.s−1. At the GC effluent, 4 psi of nitrogen was added and split 1:1 in a Gerstel 3D/2 low dead volume four-way cross (Gerstel GmbH & Co KG, Mülheim, Germany) for simultaneous flame ionization detection and EAD recording of the separated compounds. The compounds eluting from the effluent capillary for EAD were mixed with charcoal-filtered humidified air (1.5 L.min−1) in a glass tube (length 10 cm, inner diameter 6.7 mm) and released close to the prepared fly antenna. A compound was categorized as biologically active if it elicited a reproducible response in the fly antenna. All flies used for the electrophysiological studies were transferred from the plastic pipette tips to vials with 76% ethanol for subsequent confirmation of species identity by morphological analysis.
Field Trapping Experiments
To investigate whether M. signaticornis adults can be effectively attracted and collected using volatiles released by infested trees, we performed a study with available synthetic chemicals comprising 18 compounds categorized as active in GC-EAD and two additional compounds, 2-methyl-3-buten-2-ol and ipsdienol, reported to be involved in attraction of Medetera spp. (Hulcr et al. 2005, 2006).
For potential pest management application in the future it might be beneficial to attract Medetera flies to infested trees at the early stage of the beetle attack. We therefore selected synthetic mixtures of volatiles related to an early infested spruce tree for our trapping experiments. Two different quantitative compositions of the synthetic chemicals were tested: i) a 1:1 mix in which GC-EAD active and additional compounds were prepared in equal proportions, and ii) a natural mimic in which GC-EAD active and additional compounds were prepared according to the amounts released from 1 000 dm2 of an early infested standing Norway spruce tree (Table 1). Hexane (≥ 97%, Merck, Germany) was used as diluting solvent and as control.
Trapping experiments were carried out between June and August 2019 at two different locations in Sweden affected by continuous spruce bark beetle outbreaks. These were Perstorp in Halland County (56.494°N, 13.210°E) and Asa (57.150°N, 14.765°E). At both locations, we used sticky traps that consisted of cardboard rectangles (90 cm × 30 cm) with a printed spruce bark pattern covered with transparent sticky plastic foils and fixed vertically 30–40 cm above the ground on a wooden stick (Supplementary Fig. 1). The distance between traps was around 10 m. We used two traps per synthetic mixture, with three replicate sets during the experimental period. The traps were baited with dispensers consisting of a roll of dental cotton (0.5 cm outer diameter, 3.5 cm length; 2.75 cm3; DAB Dental, Upplands Väsby, Sweden) that was impregnated with 2 mL of a synthetic blend and sealed inside a low-density polyethylene sachet (LDPE; 60 × 60 mm; thickness 50 µm) (Rajapack, Gothenburg, Sweden). The dispensers were fixed in the center of the sticky traps. After 24 h, the traps were checked and the numbers of Medetera flies and I. typographus beetles were counted. Medetera flies were separated by sex based on the hypopygium (male genital apparatus) (Supplementary Fig. 2). The Medetera flies were transferred to vials with 76% ethanol for subsequent identification.
Statistical Analysis
All statistical analyses were performed in R studio (version 1.3.959) (Team 2020). Square roots of mean amounts of compounds were plotted in a heatmap using the function ggplot from the package ggplot2. To test for significant qualitative and quantitative differences between: i) volatile profiles collected from the different treatments (non-infested, infested cut, and infested standing trees); and ii) overall volatile profiles from infested samples over time, we used Permutational Multivariate Analysis of Variance (PERMANOVA, based on Bray-Curtis distances calculated from amount of compounds, 999 permutations) and pairwise PERMANOVA with Bonferroni correction for multiple testing, using the functions adonis and pairwise.factorfit from the vegan package in R (Oksanen et al. 2014). To visualize the differences in overall data collected, we used two different ordination methods: non-metric multidimensional scaling (NMDS, based on Bray–Curtis distances) from the package vegan with the function metaMDS (Bühler 2012) and principal component analysis (PCA) from the package ade4 with the function fviz_pca_ind (Oksanen et al. 2015). To identify groups of compounds found more often in one treatment or at one site compared with another, we applied multi-level pattern analysis with the multipatt function from the indicspecies package (De Cáceres et al. 2010).
We used one-way analysis of variance (ANOVA) with the function aov to compare the number of flies collected in the traps with synthetic blends. We performed post-hoc tests with emmeans for pairwise multiple comparisons.
Results
Volatile Collection and Analysis
A total of 118 compounds were found in 63 headspace collections from non-infested trees, infested standing trees, and infested cut trees (Supplementary Table 2). Figure 2 shows 56 of these compounds, 37 that were tentatively identified and 19 that were effectively identified. The overall volatile profiles differed significantly between non-infested and infested samples (PERMANOVA, P = 0.001). Within the samples from infested trees, the overall volatile profiles also differed between standing and cut trees (pairwise PERMANOVAs, all Bonferroni-corrected, P = 0.01) and changed significantly over time (PERMANOVA, P = 0.001). In contrast, volatile profiles of samples from non-infested trees did not differ over time (PERMANOVA, P > 0.1). When represented with NMDS, the overall headspace samples from non-infested trees were clearly separated from those from infested trees, while the samples from infested standing and infested cut trees were grouped together (Fig. 3A). This indicates that headspace samples collected from infested standing and cut trees were more similar, both qualitatively and quantitatively, than headspace samples collected from non-infested trees. Similar results were obtained in the PCA plot (Fig. 3B). However, principal component 1 (PC1), which discriminated between the treatments (42.9%), also showed that headspace samples grouped at the left-hand side of PC1 (mainly samples of from infested cut trees) displayed much higher amounts of compounds than samples grouped at the right-hand side of PC1 (Figs. 2 and 3B).
Abundance (square root of the mean amount released per surface area and time (ng(dm2.s)−1)) of compounds detected in the headspace samples collected from cut Norway spruce trees (Piceae abies) infested by spruce bark beetles (Ips typographus), standing infested or non-infested trees. Odors were sampled from trees at two forest sites (S1 and S2) in up to seven sequential collections (C1 to C7) from 10th May (for S1) or 25th May (for S2), respectively, to 25th July 2018. The dates for the first headspace collections C1 differed slightly between sites as bark beetles were earlier active at S1 compared to S2. However, collections C1 to C6 from sites 1 and 2 correspond to similar stages of bark beetle attack. The compounds are listed in the order of their GC retention time. Asterisks (*) indicates significant differences in the abundance of compounds between the treatments (Multipatt, *P ≤ 0.05; **P < 0.01; ***P < 0.001); n describes the number of trees used in each site per treatment. Compounds in bold have been effectively identified with synthetic standards
Comparison between the overall volatile profiles found in the headspace samples collected from cut Norway spruce trees (Piceae abies) infested by bark beetles (Ips typographus) (c), standing infested (s) or non-infested trees (u) over time. A) Non-metric multidimensional scaling (NMDS) with two synthetic axes and a stress value lower than 0.09. B and C) Principal component analysis (PCA) with PC1 and PC2 summarizing 56.1% of the variance of the dataset. Individual samples from the differently treated trees illustrated in A and B are labeled with different colors representing bark beetle-infested cut trees (blue, n = 25), bark beetle infested standing trees (yellow, n = 26) and non-infested trees (grey, n = 12). Numbers on the color-labeled points C1-7 designate the order of individual collections within the three treatments. Samples that are positioned close to each other have similar volatile profiles. The variable plot C) represents the contribution of individual compounds to the main variance in the dataset. Positively correlated compounds are grouped in the same quadrant; negatively correlated compounds are grouped in opposite quadrants. Compounds with long distance from the origin (long arrows) strongly contribute to the samples loaded in the same quadrant. Compounds represented in brown contribute significantly to the overall odor profiles at the beginning of the bark beetle attack (C1-C2) and compounds represented in green contribute significantly to the overall odor profiles at the end of bark beetle attack (C5-C7) (Multipatt, *P ≤ 0.05; **P < 0.01; ***P < 0.001)
The overall volatile profile for non-infested trees, compared with infested trees differed in terms of amounts of released compounds (e.g., camphene, cumene, α-terpinene, p-cymene, α-terpineol, terpinen-4-ol, camphor, pinocamphone, borneol, and verbenone) (Fig. 2).
Among the headspace samples from infested trees, samples from cut trees contained significantly more hydrocarbon monoterpenes (e.g., camphene, isoterpinolene, and α-terpinene), oxygenated monoterpenes (e.g., camphor, borneol, fenchone, pinocamphone, terpinen-4-ol, pinocarvone, myrtenal, and trans-pinocarveol), and aldehydes (e.g., nonanal) compared with samples from standing trees (Multipatt, P < 0.05) (Fig. 2, Supplementary Table 3). Volatile compounds such as fenchol, bornyl acetate, and geranyl acetone were only found in samples collected from standing trees, while caryophyllene oxide and cis–trans carveol were only found in samples collected from cut trees (Fig. 2).
Pairwise comparisons of infested samples collected at different time-points showed that samples collected during late bark beetle attack phases (C5-C7) differed significantly from samples collected during earlier attack phases (C1-C2) (pairwise PERMANOVA, all Bonferroni-corrected, P = 0.01). This was confirmed by the NMDS and PCA plots (Fig. 3A, B), with samples collected at the beginning of the attack (C1-C2) mainly situated in the lower, negative part of both NMDS2 (Fig. 3A) and PC2 (Fig. 3B). On the other hand, samples collected later during the attack (C5-C7) clustered more in the upper positive part of both NMDS2 (Fig. 3A) and PC2 (Fig. 3B).
The variable correlation plot resulting from the PCA (Fig. 3C) illustrates the contribution of each compound to the variation in overall dataset (including all samples collected from non-infested and infested trees at different time-points). Compounds in the lower part of PC2 (brown in Fig. 3C), such as α and β-caryophyllene, α-thujene, β-myrcene, and β-phellandrene, were emitted in larger amounts at the beginning of bark beetle attack, while compounds in the upper part of PC2 (green in Fig. 3C), such as pinocarvone, myrtenal, fenchone, β-pinone, and verbenone, were emitted in larger amounts at the end of the attack (Multipatt, both P < 0.05). This indicates that emission of hydrocarbon monoterpenes was high at the beginning of bark beetle attack and that emission of oxygenated monoterpenes was high at the end of the attack (Fig. 2; Supplementary Table 3). The shift in the overall volatile composition of infested trees seemed to occur during the first 30–40 days after bark beetle attack was initiated.
We also found a significant interaction between treatment and site (PERMANOVA, P = 0.02). The reason was that odor samples from infested cut trees collected at Site 1 contained higher amounts of hydrocarbon and oxygenated monoterpenes than samples from infested cut trees at Site 2 (Multipatt, P < 0.05) (Fig. 2, Supplementary Table 3).
GC-EAD Responses by Medetera signaticornis to Early-infested Standing Spruce Odor Samples
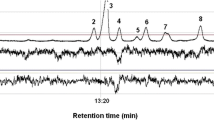
GC-EAD analysis revealed that 22 active compounds from early-infested spruce trees elicited similar antennal responses in both M. signaticornis males and females. The active compounds were identified and divided into three groups according to their primary source of origin (Fig. 4A, B). The first group comprised (–)-cis-verbenol, ( +)-trans-verbenol, and myrtenol, which are known as compounds produced by I. typographus (Birgersson et al. 1984; Birgersson 1989). The second group comprised isoterpinolene, α-pinene oxide, camphor, pinocamphone, terpinen-4-ol, myrtenal, borneol, α-terpineol, verbenone and geranyl acetone, all of which except verbenene are oxygenated monoterpenes that are known to be primarily produced by microorganisms associated with I. typographus (Leufvén et al. 1984, 1988; Kandasamy et al. 2021). The third group comprised α-pinene, α-fenchene, β-pinene, camphene, 3-carene, limonene, γ-terpinene and terpinolene, which are hydrocarbon monoterpenes produced by the spruce host tree (Phillips and Croteau 1999; Keeling and Bohlmann 2006). We found that α-terpinene elicited antennal responses in some female M. signaticornis, but not consistently in all replicates. However, the antennal activity of this compound was confirmed later with a synthetic standard (data not shown).
GC-EAD responses of M. signaticornis fly antennae stimulated with odors collected from early infested Norway spruce tree. A) Chromatogram and electroantennograms showing the antennal stimulation of a female and a male fly antenna in response to compounds eluting from the GC. Dotted lines connect antennal responses with chromatogram peaks of active compounds. B) Mean response (mV ± SE) to the active compounds organized according to their source. Compounds represented in brown font are produced by the bark beetle Ips typographus, compounds in black font are produced by the I. typographus associated microorganisms and compounds in green font are produced by the Norway spruce tree (Picea abies). (F) females and (M) males
Field Trapping Experiments
Traps with either of the two synthetic blends of chemicals (mix 1:1 and natural mimic) caught significantly more Medetera flies than the hexane control (F = 4.3; df = 2, 18; P < 0.05) (Table 2). The number of flies trapped was similar for the two synthetic blends. Approximately 71% of the flies identified from traps with synthetic blends were M. signaticornis females, 27% were M. signaticornis males, and 2% were M. ambigua Zetterstedt (Table 2).
The number of I. typographus beetles was also significantly higher for both synthetic blends compared with the control (F = 9.8; df = 2,18, P < 0.005). The traps baited with natural mimic collected slightly more bark beetles than the traps with the 1:1 mix, but the difference was not statistically significant.
Discussion
Our aim in this study was to identify key compounds that attract the bark beetle predator M. signaticornis to bark beetle-infested Norway spruce trees. In field trials, we demonstrated that M. signaticornis females and males were attracted to synthetic blends of compounds associated with bark beetles, their symbiotic microorganisms, and host trees. Analyses of headspace samples from infested Norway spruce trees showed that headspace composition and compound concentration varied depending on the time-point of collection, apparently following different stages of bark beetle attack (early, late).
More specifically, our analyses revealed that headspace samples from early-infested trees contained high amounts of hydrocarbon monoterpenes such as α and β-caryophyllene, α-thujene, β-myrcene, and β-phellandrene, while headspace samples from late-infested trees were mainly dominated by oxygenated monoterpenes such as pinocarvone, myrtenal, fenchone, β-pinone, and verbenone. Previous studies have also found that headspace samples from bark beetle-infested logs contain a complex mixture of volatiles that changes both qualitatively and quantitatively over the different stages of bark beetle attack (Birgersson et al. 1984; Birgersson and Bergström 1989; Pettersson and Boland 2003). At early stages, we found that headspace samples from infested Norway spruce trees consisted mainly of hydrocarbon mono- and sesquiterpenes, which are released as a result of bark beetle tunneling (Phillips and Croteau 1999; Keeling and Bohlmann 2006), while in late stages of attack release of oxygenated monoterpenes increased, due to the establishment of symbiotic microorganisms such as yeasts (Birgersson et al. 1984; Leufvén et al. 1984; Leufvén and Birgersson 1987) and fungi introduced by the bark beetles (Kandasamy et al. 2016, 2021). For example, Ophiostomatoid fungi (Endoconidiophora polonica, Grosmannia penicillata, Leptographium europhioides, Ophiostoma bicolor, O. piceae) lining the gallery walls in bark beetle-infested trees contribute to the release of oxygenated monoterpenes such as camphor, pinocamphone, borneol, and terpinen-4-ol (Kandasamy et al. 2016, 2021). In agreement with our findings, Pettersson and Boland (2003) observed that the maximum ratio of oxygenated monoterpenes occurs in later stages of beetle attack, which coincides with the presence of late instar bark beetle larvae and appears to be an important cue for parasitoids that attack bark beetles (Pettersson 2001). In our study, we also found that infested cut trees emitted significantly higher amounts of certain volatile compounds compared to infested standing trees. According to the literature, cutting induces changes in the volatile composition, such as increased release of oxygenated monoterpenes (Strömvall and Petersson 1991; Pettersson and Boland 2003). Mechanical damage increases the release of volatile terpenes from host trees that can auto-oxidize when exposed to the air generating more oxygenated monoterpenes (Keeling and Bohlmann 2006; Benoid et al. 2021). In addition, exposed wounds can be contaminated with various types of microorganisms that can contribute to the emission of compounds from cut trees. In our study, we have not measured and compared the volatiles from non-infested cut trees and for this reason it is not possible to conclude which compounds are being produced as a direct result of the mechanical damage.
According to Hedgren et al. (2004), differences in the release rates and composition of volatiles from infested standing and infested cut trees seem to affect attraction of different Medetera spp. species, with the total number of Medetera species emerging from bark beetle-infested standing Norway spruce trees being 10 times higher than the number emerging from infested cut trees. Moreover, some Medetera species were present in both standing and cut Norway spruce trees, while other species only occurred in standing or in cut infested trees. In addition to the composition and release rates of compounds from infested cut or infested standing trees, the number of prey beetles, nutritional quality of the bark and visual cues such as tree orientation, bark texture, and hardness may also influence host location and oviposition by Medetera flies (Lawson et al. 1996; Goyer et al. 2004).
Previous studies have shown that M. setiventris and M. melancholica are attracted to components of I. typographus aggregation pheromone and that the attraction increases if aggregation pheromone is combined with host tree monoterpenes (α-pinene, β-pinene, and limonene) (Rudinsky et al. 1971; Hulcr et al. 2005, 2006). However, logs from infested trees have been found to be more attractive to M. bistriata Parent adults than a mixture of bark beetle aggregation pheromone and tree monoterpenes, indicating that additional cues, such as volatile organic compounds produced by microbial bark beetle symbionts, might play a role in host location (Williamson 1971). Medetera signaticornis adults arrive at freshly attacked trees almost simultaneously with bark beetles, but have also been found on attacked trees after emission of bark beetle pheromone has ceased (Lawson et al. 1997). Like M. bistriata, M. signaticornis adults may use other reliable host cues besides bark beetle aggregation pheromone and tree compounds.
Odors emitted from microorganisms living in symbiosis with bark beetles have been shown to impact the behavior of some unidentified Medetera species, which are more attracted to logs colonized by fungi (e.g., Ophiostoma ips) or a bacterial strain (Burkholderia sp.) than to uncolonized logs (Boone et al. 2008). Individuals of M. signaticornis are commonly found on Norway spruce trees infested with I. typographus, but have also been reported on other Picea and Pinus tree species infested with bark beetles from the genera Dendroctunus, Dryocoetes, Scolytus, and Pityogenes (Coleoptera: Curculionidae, Scolytinae) (Bickel 1985). Many of these bark beetle species, if not all, are associated with Ophiostomatoid fungi (Klepzig and Six 2004). Thus, volatiles from Ophiostoma fungi combined with tree-produced compounds may provide reliable cues for the predatory M. signaticornis to detect hosts throughout a bark beetle attack, even after pheromone production by the bark beetle has ceased. Microbial odors are important components of tritrophic interactions and may contribute to the attraction or repellence of predators and parasitoids to food sources or oviposition sites (Davis et al. 2013; Kandasamy et al. 2016).
Our GC-EAD studies on odors collected from freshly attacked spruce logs revealed that M. signaticornis males and females were able to detect several compounds produced by the host trees, bark beetles, and bark beetle associated microorganisms. The flies responded to (–)-cis-verbenol, ( +)-trans-verbenol, and myrtenol. These three compounds are produced by the bark beetle I. typographus (Birgersson et al. 1984; Birgersson 1989) and are detoxification products from ( ±)-α-pinene (i.e. (–)-(4S)-cis-verbenol from (–)-α-pinene, ( +)-(4S)-trans-verbenol from ( +)-α-pinene, and myrtenol from both ( +) and (–)-α-pinene), but only (–)-cis-verbenol is known as a pheromone component by I. typographus (Renwick et al. 1976; Wood 1982; Lindström et al. 1989; Blomquist et al. 2010). The flies also responded to isoterpinolene, α-pinene oxide, camphor, pinocamphone, terpinen-4-ol, myrtenal, borneol, α-terpineol, verbenone and geranyl acetone. These compounds are known to be primarily produced by microorganisms associated with I. typographus (Leufvén et al. 1984, 1988; Kandasamy et al. 2021). However, some compounds (e.g., terpinen-4-ol, camphor, borneol, α-terpineol and verbenone) can also be found in small amounts in the different parts (e.g., needles, bark, roots) of a healthy Norway spruce tree (Duan et al. 2020) or in other plants species (e.g., pinocamphone, camphor, borneol, α-terpineol, verbenone and geranyl acetone) (Knudsen et al. 1993). Verbenone can also be produced by many species of Dendroctonus. However, Ips beetles in general, and I. typographus in particular, does not produce verbenone (Francke and Vité 1983). Therefore in this case microorganisms are the most probable source of this compound. Verbenene also included in this group is not oxygenated per se, but may be a deoxidized product of verbenone as both compounds have a similar chemical structure and according to Blomquist et al. (2010) verbenol, verbenone and verbenene are all produced from hydroxylation of α-pinene. In addition, verbenene has not been found produced by I. typographus or the host tree and therefore the most probable source is microbial.
Interestingly, many of these GC-EAD active compounds are also known to be detected by other natural enemies of I. typographus (see Supplementary Table 4). For example, the predatory clerid beetle Thanasimus formicarius Linnaeus (Coleoptera: Cleridae) is attracted to bark beetle aggregation pheromone and tree monoterpenes, and possesses olfactory receptors for oxygenated monoterpenes produced by symbiotic microorganisms (e.g., camphor and pinocamphone) (Hansen 1983; Tømmerås 1985). Similarly, the Pteromalid parasitoid species Rhopalicus tutela Walker, Roptrocerus mirus Walker, and Roptrocerus xylophagorum Ratzeburg (Hymenoptera: Pteromalidae) respond to tree-produced compounds, but seem to be more attracted to oxygenated monoterpenes (e.g., camphor and pinocamphone) primarily produced by symbiotic fungi of the bark beetle (Pettersson 2001; Pettersson et al. 2001; Pettersson and Boland 2003). The detection of such microbial odors by different classes of natural enemies indicates that these may be crucial for location of bark beetles as prey. Therefore, further studies need to be performed to determine whether specific fungal compounds are necessary or sufficient to attract natural enemies such as Medetera flies.
Our field experiments with traps baited with synthetic blends of potential host cues revealed attraction for both sexes of M. signaticornis. Unsurprisingly, females, which use spruce trees for oviposition, were attracted in higher numbers than males. It is unclear why males are attracted to bark beetle-infested trees, but they are possibly used as meeting and mating sites by both sexes of M. signaticornis (Hopping 1947). Individuals of M. ambigua were also found in the traps, indicating that attraction was not limited to M. signaticornis. In the future, we will examine in more detail the attractiveness of synthetic blends for M. signaticornis and other Medetera species.
In this study, we tested and confirmed the hypothesis that M. signaticornis adult flies use multiple semiochemicals to detect bark beetle-infested Norway spruce trees throughout infestation. Male and female flies responded both electrophysiologically and behaviorally to several compounds emitted from host trees, bark beetles, and symbiotic microorganisms. Besides tree-produced compounds, oxygenated monoterpenes produced by symbiotic microorganisms may be a reliable cue for the predatory M. signaticornis, especially in the later stages of bark beetle attack when production of bark beetle pheromone has declined. Thus, the multitrophic interaction between predatory M. signaticornis, bark beetle, host tree, and microorganisms needs to be assessed in future studies on management and ecology of bark beetles and their natural enemies. The present study provides a sound foundation for further field work aiming to adjust the attractiveness of the synthetic blend to mimic relevant host cues. Such a blend could be used to monitor Medetera flies or to attract more flies to newly infested areas, increasing biological control and reducing the number of bark beetles emerging from infested trees, which in turn would reduce the economic losses to the forest sector.
Data Availability
All of the data on which conclusions rely in this study are included in this published article and its supplementary information files.
References
Anon (2023) Reference climate data from the Unit for Field-based Forest Research climate monitoring program. University of Agricultural Sc. [Downloaded from https://www.slu.se/esf-referenceclimate/, 2023-01-10]
Beaver RA (1966) The biology and immature stages of two species of Medetera (Diptera: Dolichopodidae) associated with the bark beetle Scolytus scolytus (F.). Proceedings of the Royal Entomological Society of London. Series A, General Entomology. Wiley Online Library, pp 145–154. https://doi.org/10.1111/j.1365-3032.1966.tb00334.x
Benoid R, Belhadj N, Lailliau M, Dagaut P (2021) Autoxidation of terpenes a common pathway in trophospheric and low temperature combustion conditions: the case of limonene and α-pinene. Atmos Chem Phys Discuss [preprint]. https://doi.org/10.5194/acp-2021-964
Bentz BJ, Jönsson AM, Schroeder M, Weed A, Wilcke RAI, Larsson K (2019) Ips typographus and Dendroctonus ponderosae models project thermal suitability for intra-and inter-continental establishment in a changing climate. Front Forests Global Change 2:1. https://doi.org/10.3389/ffgc.2019.00001
Bickel DJ (1985) A revision of the nearctic Medetera (Diptera: Dolichopodidae), United States Department of Agriculture, Agricultural Research Service. Technical Bulletin Number 1692
Birgersson G (1989) Host tree resistance influencing pheromone production in Ips typographus (Coleoptera: Scolytidae). Ecography 12:451–456. https://doi.org/10.1111/j.1600-0587.1989.tb00922.x
Birgersson G, Bergström G (1989) Volatiles released from individual spruce bark beetle entrance holes quantitative variations during the first week of attack. J Chem Ecol 15:2465–2483. https://doi.org/10.1007/BF01020377
Birgersson G, Schlyter F, Löfqvist J, Bergström G (1984) Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J Chem Ecol 10:1029–1055. https://doi.org/10.1007/BF00987511
Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, Chang E, Tittiger C (2010) Pheromone production in bark beetles. Insect Biochem Mol Biol 40:699–712. https://doi.org/10.1016/j.ibmb.2010.07.013
Boone CK, Six DL, Zheng Y, Raffa KF (2008) Parasitoids and dipteran predators exploit volatiles from microbial symbionts to locate bark beetles. Environ Entomol 37:150–161. https://doi.org/10.1093/ee/37.1.150
Bühler A (2012) NMDS Tutorial in R. https://jonlefcheck.net/2012/10/24/nmds-tutorial-in-r/. Accessed 22 Aug 2022
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859. https://doi.org/10.1007/s10886-013-0306-z
De Cáceres M, Legendre P, Moretti M (2010) Improving indicator species analysis by combining groups of sites. Oikos 119:1674–1684. https://doi.org/10.1111/j.1600-0706.2010.18334.x
Duan Q, Bonn B, Kreuzwieser J (2020) Terpenoids are transported in the xylem sap of Norway spruce. Plant Cell Environ 43:1766–1778. https://doi.org/10.1111/pce.13763
Edmonds RL, Eglitis A (1989) The role of the Douglas-fir beetle and wood borers in the decomposition of and nutrient release from Douglas-fir logs. Can J for Res 19:853–859. https://doi.org/10.1139/x89-130
El-Sayed A (2016) The pherobase: database of pheromones and semiochemicals. https://www.pherobase.com/database/compound/compounds-index.php. Accessed Apr 2022
Fitzgerald T, Nagel W (1972) Oviposition and larval bark-surface orientation of Medetera aldrichii (Diptera: Dolichopodidae): response to a prey-liberated plant terpene. Ann Entomol Soc Am 65:328–330. https://doi.org/10.1093/aesa/65.2.328
Francke W, Vité JP (1983) Oxygenated monoterpenes in pheromone systems of bark beetles 1. Zeitschrift Für Angew Entomol 96:146–156. https://doi.org/10.1111/j.1439-0418.1983.tb03655.x
Ghimire RP, Kivimäenpää M, Blomqvist M, Holopainen T, Lyytikäinen-Saarenmaa P, Holopainen JK (2016) Effect of bark beetle (Ips typographus L.) attack on bark VOC emissions of Norway spruce (Picea abies Karst.) trees. Atmos Environ 126:145–152. https://doi.org/10.1016/j.atmosenv.2015.11.049
Gomez-Gallego M, Galiano L, Martínez-Vilalta J, Stenlid J, Capador-Barreto HD, Elfstrand M, Camarero JJ, Olivia J (2022) Interaction of drought- and pathogen-induced mortality in Norway spruce and Scots pine. Plant Cell Environ 45:2292–2305. https://doi.org/10.1111/pce.14360
Goyer R, Lenhard G, Strom BL (2004) The influence of silhouette color and orientation on arrival and emergence of Ips pine engravers and their predators in loblolly pine. For Ecol Manag 191:147–155. https://doi.org/10.1016/j.foreco.2003.11.012
Grégoire J-C, Raffa KF, Lindgren BS (2015) Bark Beetles: Biology and Ecology of Native and Invasive Species. Chapter 1, Elsevier, pp 1–40. https://doi.org/10.1016/B978-0-12-417156-5.00001-0
Gunulf A, Wang L, Englund J-E, Rönnberg J (2013) Secondary spread of Heterobasidion parviporum from small Norway spruce stumps to adjacent trees. For Ecol Manag 287:1–8. https://doi.org/10.1016/j.foreco.2012.09.011
Hammerbacher A, Schmidt A, Wadke N, Wright LP, Schneider B, Bohlmann J, Brand WA, Fenning TM, Gershenzon J, Paetz C (2013) A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol 162:1324–1333. https://doi.org/10.1104/pp.113.218610
Hannerz M, Thorsén Å, Mattsson S, Weslien J (2002) Pine weevil (Hylobius abietis) damage to cuttings and seedlings of Norway spruce. For Ecol Manag 160:11–17. https://doi.org/10.1016/S0378-1127(01)00467-4
Hansen K (1983) Reception of bark beetle pheromone in the predaceous clerid beetle, Thanasimus formicarius (Coleoptera: Cleridae). J Comp Physiol 150:371–378. https://doi.org/10.1007/BF00605026
Hedgren PO, Schroeder LM (2004) Reproductive success of the spruce bark beetle Ips typographus (L.) and occurrence of associated species: a comparison between standing beetle-killed trees and cut trees. For Ecol Manag 203:241–250. https://doi.org/10.1016/j.foreco.2004.07.055
Hlásny T, Zimova S, Merganicova K, Stepanek P, Modlinger R, Turcani M (2021) Devastating outbreak of bark beetles in the Czech Republic: drivers, impacts, and management implications. For Ecol Manag 490:119075. https://doi.org/10.1016/j.foreco.2021.119075
Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimäenpää M (2018) Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front Plant Sci 9:1–10. https://doi.org/10.3389/fpls.2018.01445
Hopping GR (1947) Notes on the seasonal development of Medetera aldrichii Wheeler (Diptera: Dolichopodidae) as a predator of the douglas fir bark-beetle, Dendroctonus Pseudotsugae Hopkins L. Can Entomol 79:150–153. https://doi.org/10.4039/Ent79150-7
Hulcr J, Pollet M, Ubik K, Vrkoc J (2005) Exploitation of kairomones and synomones by Medetera spp.(Diptera: Dolichopodidae), predators of spruce bark beetles. Eur J Entomol 102:655–662
Hulcr J, Ubik K, Vrkoc J (2006) The role of semiochemicals in tritrophic interactions between the spruce bark beetle Ips typographus, its predators and infested spruce. J Appl Entomol 130:275–283. https://doi.org/10.1111/j.1439-0418.2006.01069.x
Juráň S, Pallozzi E, Guidolotti G, Fares S, Šigut L, Calfapietra C, Alivernini A, Savi F, Večeřová K, Křůmal K, Večeřa Z, Urban O (2017) Fluxes of biogenic volatile organic compounds above temperate Norway spruce forest of the Czech Republic. Agr for Meteorol 232:500–513. https://doi.org/10.1016/j.agrformet.2016.10.005
Kandasamy D, Gershenzon J, Hammerbacher A (2016) Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J Chem Ecol 42:952–969. https://doi.org/10.1007/s10886-016-0768-x
Kandasamy D, Zaman R, Nakamura Y, Zhao T, Hartmann H, Andresson MN, Hammerbacher A, Gershenzon J (2021) Bark beetles locate fungal symbionts by detecting volatile fungal metabolites of host tree resin monoterpenes. BioRxiv. https://doi.org/10.1101/2021.07.03.450988
Kärvemo S, Schroeder LM (2010) A comparison of outbreak dynamics of the spruce bark beetle in Sweden and the mountain pine beetle in Canada (Curculionidae: Scolytinae). Ent Tidskr 131:215–224
Keeling CI, Bohlmann J (2006) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol 170:657–675. https://doi.org/10.1111/j.1469-8137.2006.01716.x
Kenis M, Wermelinger B, Grégoire J-C (2007) Research on Parasitoids and Predators of Scolytidae – A Review. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-2241-8_11
Klepzig KD, Six D (2004) Bark beetle-fungal symbiosis: context dependency in complex associations. Symbiosis 37:189–205
Knudsen JT, Tollsten L, Bergström G (1993) Floral scents – a checklist of volatile compounds isolated by headspace techniques. Phytochemistry 33:253–280
Lawson SA, Furuta K, Katagiri K (1996) The effect of host tree on the natural enemy complex of Ips typographus japonicus Niijima (Col., Scolytidae) in Hokkaido, Japan. J Appl Entomol 120:77–86. https://doi.org/10.1111/j.1439-0418.1996.tb01570.x
Lawson SA, Furuta K, Katagiri K (1997) Effect of natural enemy exclusion on mortality of Ips typographus japonicus Niijima (Col., Scolytidae) in Hokkaido, Japan. J Appl Entomol 121:89–98. https://doi.org/10.1111/j.1439-0418.1997.tb01376.x
Leufvén A, Birgersson G (1987) Quantitative variation of different monoterpenes around galleries of Ips typographus (Colleoptera: Scolytidae) attacking Norway spruce. Canad J Bot 65:1038–1044. https://doi.org/10.1139/b87-144
Leufvén A, Bergström G, Falsen E (1984) Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J Chem Ecol 10:1349–1361. https://doi.org/10.1007/BF00988116
Leufvén A, Bergström G, Falsen E (1988) Oxygenated monoterpenes produced by yeasts, isolated from Ips typographus (Coleoptera: Scolytidae) and grown in phloem medium. J Chem Ecol 14:353–362. https://doi.org/10.1007/BF01022551
Lindström M, Torbjörn N, Göran B, Fredrik S (1989) Variation of enantiomeric composition of α-pinene in norway spruce, Picea abies, and its influence on production of verbenol isomers by Ips typographus in the field. J Chem Ecol 15:541–548. https://doi.org/10.1007/BF01014699
Marini L, Økland B, Jönsson AM, Bentz B, Carroll A, Forster B, Grégoire JC, Hurling R, Nageleisen LM, Netherer S (2017) Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 40:1426–1435. https://doi.org/10.1111/ecog.02769
Martin DM, Gerhenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant physiol 132:1586–1599 (http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.021196)
Nicolai V (1995) The impact of Medetera dendrobaena Kowarz (Dipt., Dolichopodidae) on bark beetles. J Appl Entomol 119:161–166. https://doi.org/10.1111/j.1439-0418.1995.tb01264.x
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’Hara R, Simpson G, Solymos P, Stevens M, Wagner H (2014) Vegan: Community ecology package. R package version 2.2-.0. http://CRAN.Rproject.org/package=vegan. Accessed May 2022
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’Hara R, Simpson G, Solymos P, Henry M, Stevens M (2015) Vegan community ecology package: ordination methods, diversity analysis and other functions for community and vegetation ecologists. R package 2.6–2. http://CRAN.Rproject.org/package=vegan. Accessed May 2022
Ounap H (2001) Insect predators and parasitoids of bark beetles (Col., Scolytidae) in Estonia. PhD Thesis, Institute of Plant Protection, Faculty of Agronomy, Estonian Agricultural University, Estonia
Pettersson EM (2001) Volatile attractants for three Pteromalid parasitoids attacking concealed spruce bark beetles. Chemoecology 11:89–95. https://doi.org/10.1007/PL00001837
Pettersson EM, Boland W (2003) Potential parasitoid attractants, volatile composition throughout a bark beetle attack. Chemoecology 13:27–37. https://doi.org/10.1007/s000490300003
Pettersson EM, Birgersson G, Witzgall P (2001) Synthetic attractants for the bark beetle parasitoid Coeloides bostrichorum Giraud (Hymenoptera: Braconidae). Naturwissenschaften 88:88–91. https://doi.org/10.1007/s001140100209
Pettersson EM (2000) Vital volatiles-host location in parasitic wasps attacking bark beetles. PhD Thesis, Institute of Chemical Ecology, University of Gothenburg, Sweden
Phillips MA, Croteau RB (1999) Resin-based defenses in conifers. Trends Plant Sci 4:184–190. https://doi.org/10.1016/s1360-1385(99)01401-6
Renwick J, Hughes P, Krull I (1976) Selective production of cis-and trans-verbenol from (-)-and (+)-α-pinene by a bark beetle. Science 191:199–201. https://doi.org/10.1126/science.1246609
Romashkin I, Neuvonen S, Tikkanen O-P (2020) Northward shift in temperature sum isoclines may favour Ips typographus outbreaks in European Russia. Agric For Entomol 22:238–249. https://doi.org/10.1111/afe.12377
Rouault G, Candau J-N, Lieutier F, Nageleisen L-M, Martin J-C, Warzée N (2006) Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann for Sci 63:613–624. https://doi.org/10.1051/forest:2006044
Rudinsky J, Novak V, Švihra P (1971) Attraction of the Bark Beetle Ips typographus L. to terpenes and a male-produced pheromone. J Appl Entomol 67:179–188
Schlyter F, Birgersson G, Byers JA, Löfqvist J, Bergström G (1987) Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J Chem Ecol 13:701–716. https://doi.org/10.1007/BF01020153
Seybold SJ, Huber DPW, Lee JC, Graves AD, Bohlmann J (2006) Pine monoterpenes and pine bark beetle: a marriage of convenience for defense and chemical communication. Phytochem Rev 5:143–178. https://doi.org/10.1007/s11101-006-9002-98
Stadelmann G, Bugmann H, Wermelinger B, Bigler C (2014) Spatial interactions between storm damage and subsequent infestations by the European spruce bark beetle. For Ecol Manag 318:167–174. https://doi.org/10.1016/j.foreco.2014.01.22
Strömvall AM, Petersson G (1991) Conifer monoterpenes emitted to air by logging operations. Scand J for Res 6:253–258. https://doi.org/10.1080/02827589109382666
Team R (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston URL http://www.rstudio.com/. Accessed Apr 2022
Tømmerås BÅ (1985) Specialization of the olfactory receptor cells in the bark beetle Ips typographus and its predator Thanasimus formicarius to bark beetle pheromones and host tree volatiles. J Comp Physiol 157:335–341. https://doi.org/10.1007/BF00618123
Ulrich H (2004) Predation by adult Dolichopodidae (Diptera): a review of literature with an annotated prey-predator list. Studia Dipterologica 11(2):369–403
Wadke N, Kandasamy D, Vogel H, Lah L, Wingfield BD, Paetz C, Wright LP, Gershenzon J, Hammerbacher A (2016) The bark-beetle-associated fungus Endoconidiophora polonica, utilizes the phenolic defense compounds of its host as a carbon source. Plant Physiol 171:914–931. https://doi.org/10.1104/pp.15.01916
Wegensteiner R, Wermelinger B, Herrmann M (2015) Bark Beetles: Biology and Ecology of Native and Invasive Species. Chapter 7, Elsevier, pp 247–304. https://doi.org/10.1016/B978-0-12-417156-5.00007-1
Wermelinger B (2002) Development and distribution of predators and parasitoids during two consecutive years of an Ips typographus (Col., Scolytidae) infestation. J Appl Entomol 126:521–527. https://doi.org/10.1046/j.1439-0418.2002.00707.x
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For Ecol Manag 202:67–82. https://doi.org/10.1016/j.foreco.2004.07.018
Williamson D (1971) Olfactory Discernment of Prey by Medetera bistriata (Diptera: Dolichopodidae). Ann Entomol Soc Am 64:586–589
Wood DL (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Ann Rev Entomol 27:411–446. https://doi.org/10.1146/annurev.en.27.010182.002211
Zhao T, Kandasamy D, Krokene P, Chen J, Gershenzon J, Hammerbacher A (2019) Fungal associates of the tree-killing bark beetle, Ips typographus vary in virulence, ability to degrade and influence bark beetle tunneling behavior. Fungal Ecol 38:71–79. https://doi.org/10.1016/j.funeco.2018.06.003
Acknowledgements
The authors would like to thank Martin Ahlström from SLU Field Research Station in Asa and Annette Johansson from SnifferDogs Sweden for help and advice at the forest sites, Adam Flöhr for statistical support. Isabella Kleman and Antonio Giannuzzi for field work assistance. The Swedish research council Formas (grant number 942-2015-1335) and the SLU Center for Biological Control supported the project economically.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. This work was supported by the Swedish research council Formas (grant number 942–2015-1335) and the SLU Center for Biological Control.
Author information
Authors and Affiliations
Contributions
Maria Sousa, Paul G. Becher, Kristina Karlsson Green and Göran Birgersson planned and conceived the study. Maria Sousa collected, analysed the data and wrote the original draft of the manuscript and all authors commented on previous versions of the manuscript. Marc Pollet identified the specimens and gave guidance during the early stage of the study. Göran Birgersson initiated the project. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sousa, M., Birgersson, G., Karlsson Green, K. et al. Odors Attracting the Long-Legged Predator Medetera signaticornis Loew to Ips typographus L. Infested Norway Spruce Trees. J Chem Ecol 49, 451–464 (2023). https://doi.org/10.1007/s10886-023-01405-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-023-01405-6