Abstract
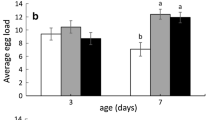
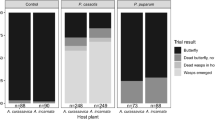
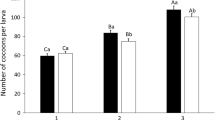
Sequestration of plant compounds by herbivorous insects as a defense against predators is well documented; however, few studies have examined the effectiveness of sequestration as a defense against parasitoids. One assumption of the “nasty host” hypothesis is that sequestration of plant defense compounds is deleterious to parasitoid development. We tested this hypothesis with larvae of the sequestering sphingid Ceratomia catalpae, which is heavily parasitized by the endoparasitoid Cotesia congregata, despite sequestering high concentrations of the iridoid glycoside catalpol from their catalpa host plants. We collected C. catalpae and catalpa leaves from six populations in the Eastern US, and allowed any C. congregata to emerge in the lab. Leaf iridoid glycosides and caterpillar iridoid glycosides were quantified, and we examined associations between sequestered caterpillar iridoid glycosides and C. congregata performance. Caterpillar iridoid glycosides were not associated with C. congregata field parasitism or number of offspring produced. Although wasp survival was over 90% in all populations, there was a slight negative relationship between caterpillar iridoid glycosides and wasp survival. Iridoid glycosides were present in caterpillars at levels that are deterrent to a variety of vertebrate and invertebrate predators. Thus, our results support the alternative hypothesis that unpalatable, chemically defended hosts are “safe havens” for endoparasitoids. Future trials examining the importance of catalpol sequestration to potential natural enemies of C. congregata and C. catalpae are necessary to strengthen this conclusion.




Similar content being viewed by others
References
Baerg, W.J. 1935. Three shade tree insects, II. Great elm leaf beetle, catalpa sphinx, and eastern tent caterpillar. Univ. Ark. Exp. Station Bull. 317: 27.
Baur, M.E. and Yeargan, K.V. 1994. Developmental stages and kairomones from the primary parasitoid Cotesia marginiventris (Hymenoptera, Braconidae) affect the response of the hyperparasitoid Mesochorus discitergus (Hymenoptera, Ichneumonidae) to parasitized caterpillars. Ann. Entomol. Soc. Am. 87: 954–961.
Beckage, N.E. and Tan, F.F. 2002. Development of the braconid wasp Cotesia congregata in a semi-permissive noctuid host, Trichoplusia ni. J. Invert. Path. 81:49–52.
Bowers, M.D. 1980. Unpalatability as a defense strategy of Euphydryas phaeton (Lepidoptera, Nymphalidae). Evolution 34: 586–600.
Bowers, M.D. 1981. Unpalatability as a defense strategy of Western checkerspot butterflies (Euphydryas Scudder, Nymphalidae). Evolution 35: 367–375.
Bowers, M.D. 1990. Recycling plant natural products for chemical defense. pp. 353–386 in D.L. EVANS (ed) Insect Defenses. State University of New York Press, Albany, NY, USA.
Bowers, M.D. 1991. Iridoid Glycosides. pp. 297–327 in G. ROSENTHAL and M. BERENBAUM (eds). Herbivores: Their Interactions with Secondary Plant Metabolites, Vol. 1, 2nd ed. Academic Press, Inc.
Bowers, M.D. 1992. The evolution of unpalatability and the cost of chemical defense in insects. pp. 216–244 in B.D. ROITBERG and M.B. ISMAN (eds) Insect Chemical Ecology: An Evolutionary Approach. Chapman & Hall, New York, NY.
Bowers, M.D. 1993. Aposematic caterpillars: life-styles of the warningly colored and unpalatable. pp. 331–371 in N.E. STAMP, T.M. CASEY (eds) Caterpillars: Ecological and Evolutionary Constraints on Foraging. Chapman & Hall, New York, NY.
Bowers, M.D. 2003. Hostplant suitability and defensive chemistry of the catalpa sphinx Ceratomia catalpae (Lepidoptera: Sphingidae). J. Chem. Ecol. 29: 2359–2367.
Bowers, M.D. and Puttick, G.M. 1986. Fate of ingested iridoid glycosides in lepidopteran herbivores. J. Chem. Ecol. 12: 169–178.
Bowers, M.D. and Puttick, G.M. 1988. Response of generalist and specialist insects to qualitative allelochemical variation. J. Chem. Ecol. 14: 319–334.
Bowers, M.D. and Puttick, G.M. 1989. Iridoid glycosides and insect feeding preferences—gypsy moths (Lymantria dispar, Lymantriidae) and buckeyes (Junonia coenia, Nymphalidae). Ecol. Entomol. 14: 247–256.
Camara, M.D. 1997. Predator responses to sequestered plant toxins in buckeye caterpillars: are tritrophic interactions locally variable? J. Chem. Ecol. 23: 2093–2106.
Campbell, B.C. and Duffey, S.S. 1979. Tomatine and parasitic wasps—potential incompatibility of plant antibiosis with biological-control. Science 205: 700–702.
Cornell, H.V. and Hawkins, B.A. 1995. Survival patterns and mortality sources of herbivorous insects—some demographic trends. Am. Nat. 145: 563–593.
Crocker, V.M. 2008. Behavioral and Developmental Responses of the Parasitoid, Cotesia congregata (Say) Differ with Respect to Plant-Host Origin: a Test for Local Adaptation. M.S. Thesis. Virginia Commonwealth University.
De La Fuente, M.A., Dyer, L.A., and Bowers, M.D. 1994/1995. The iridoid glycoside, catalpol, as a deterrent to the predator Camponotus floridanus (Formicidae). Chemoecology 5/6: 13–18.
Duffey, S.S. 1980. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25: 447–477.
Dyer, L.A. 1995. Tasty generalists and nasty specialists—antipredator mechanisms in tropical lepidopteran larvae. Ecology 75: 1483–1496.
Dyer, L.A., Bowers, M.D. 1996. The importance of sequestered iridoid glycosides as a defense against an ant predator. J. Chem. Ecol. 22: 1527–1539.
Dyer, L.A. and Gentry, G.L. 1999. Predicting natural-enemy responses to herbivores in natural and managed ecosystems. Ecol. App. 9: 402–408.
Gaines, D.N. and Kok, L.T. 1999. Impact of hyperparasitoids on Cotesia glomerata in Southwestern Virginia. Biol. Cont. 14: 19–28.
Gauld, I.D., Gaston, K.J., and Janzen, D.H. 1992. Plant allelochemicals, tritrophic interactions and the anomalous diversity of tropical parasitoids: the “nasty” host hypothesis. Oikos 65: 353–357.
Gauld, I.D., Gaston, K.J., Hawkins, B.A., and Sheehan, W. 1994. The taste of enemy-free space: parasitoids and nasty hosts. pp. 279-299 in Parasitoid Community Ecology. Oxford University Press, New York.
Gentry, G.L. and Dyer, L.A. 2002. On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 83: 3109–3119.
GODFRAY, H.C.J. 1994. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press, Princeton, NJ.
Harvey, J.A., Van Nouhuys, S., and Biere, A. 2005. Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J. Chem. Ecol. 31: 287–302.
Harwood, S.G., Mcelfresh, J.S., Nguyen, A., Conlan, C.A., and Beckage, N.E. 1998. Production of early expressed parasitism-specific proteins in alternate sphingid hosts of the braconid wasp Cotesia congregata. J. Invert. Path. 71:271–279.
Krombein, K. V., Hurd, P. D., Smith, D.R., and Burkes B.D. (eds). 1979. Catalog of Hymenoptera in America North of Mexico. Vol 1, Smithsonian Institution Press.
Lampert, E.C., Zangerl, A.R., Berenbaum, M.R., and Ode, P.J. 2008. Tritrophic effects of xanthotoxin on the polyembryonic parasitoid Copidosoma sosares (Hymenoptera: Encyrtidae). J. Chem. Ecol. 34: 783–790.
Mcdougall, C, Philogen, B.J.R., Arnason, J.T., and Donskov, N. 1988. Comparative effects of two plant secondary metabolites on host-parasitoid association. J. Chem. Ecol. 14: 1239–1252.
Nayar, J.K. and Fraenkel, G. 1963. The chemical basis of the host selection in the Catalpa sphinx, Ceratomia catalpae (Lepidoptera, Sphingidae). Ann. Entomol. Soc. Am. 56: 119–122.
Ness, J.H. 2003a. Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 134: 210–218.
Ness, J.H. 2003b. Contrasting exotic Solenopsis invicta and native Forelius pruinosus ants as mutualists with Catalpa bignonioides, a native plant. Ecol. Entomol. 28: 247–251.
Nieminen, M., Suomi, J., Van Nouhuys S., Sauri, P., and Riekkola, M. 2003. Effect of iridoid glycoside content on oviposition host plant choice and parasitism in a specialist herbivore. J. Chem. Ecol. 29: 823-844.
Nishida, R. 2002. Sequestration of defensive substance from plants by Lepidoptera. Annu. Rev. Entomol. 47: 57–92.
Opitz, S.E.W. and Müller, C. 2009. Plant chemistry and insect sequestration. Chemoecology 19: 117–154.
QUICKE, D.L.J. 1997. Parasitic Wasps. Chapman & Hall, London.
Rayor, L.S. and Munson, S. 2002. Larval feeding experience influences adult predator acceptance of chemically defended prey. Entomol. Exp. App. 104: 193–201.
Reudler Talsma, J. 2007. Costs and benefits of iridoid glycosides in multitrophic systems. Ph.D. dissertation. Netherland Institute for Ecology.
Sime, K. 2002. Chemical defense of Battus philenor larvae against attack by the parasitoid Trogus pennator. Ecol. Entomol. 27: 337–345.
Singer, M.S. and Stireman, J.O. 2003. Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 100: 554–652.
Smilanich, A.M., L.A. Dyer, J.Q. Chambers, and M.D. Bowers. 2009 Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol. Lett. 12: 612–621.
Strohmeyer, H.H., Stamp, N.E., and Bowers, M.D. 1998. Prey species and prey diet affect growth of invertebrate predators. Ecol. Entomol. 23: 68–79.
Theodoratus, D.H. and Bowers, M.D. 1999. Effects of sequestered iridoid glycosides on prey choice of the wolf spider Lycosa carolinensis. J. Chem. Ecol. 25:283–295.
Von Poser, G.L., Schripsema, J., Henriques, A.T., and Jensen, S.R., 2000. The distribution of iridoids in Bignoniaceae. Biochem. Syst. Ecol. 28:351–366.
Zvereva, E.L. and Rank, N.E. 2003. Host plant effects on parasitoid attack on the leaf beetle Chrysomela lapponica. Oecologia 135: 258–267.
Acknowledgements
We thank Karen Kester and Vanessa Crocker, Department of Biology, Virginia Commonwealth University; Richard Olsen, USDA-ARS, Washington DC, and the U.S. National Arboretum; and Clyde Sorenson, Department of Entomology, NC State University for advice on catalpa sphinx locations and Laura McLoud for shipping caterpillars from Clemson, SC. Ellen Brown of the Reynolds Homestead Historical Site, Ernest and Fred Tyson, and David Pace permitted collections on private property. Richard Olsen, Carolina Quintero, Susan Whitehead, and two anonymous reviewers provided useful comments on the manuscript. This study was funded by NSF grant DEB 0614883 to MDB and LAD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lampert, E.C., Dyer, L.A. & Bowers, M.D. Caterpillar Chemical Defense and Parasitoid Success: Cotesia congregata Parasitism of Ceratomia catalpae . J Chem Ecol 36, 992–998 (2010). https://doi.org/10.1007/s10886-010-9840-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9840-0