Abstract
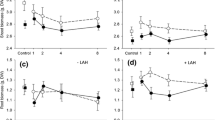
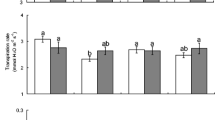
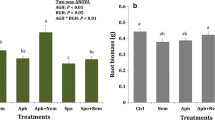
Herbivores induce systemic changes in plant traits, and the strength of these induced responses is often associated with the degree of vascular connectivity that links damaged and undamaged plant tissues. Although this phenomenon is known to occur aboveground in leaves, it is unknown whether or not leaf–root induction similarly follows the vascular architecture of plants. To test for this possibility, we manipulated foliar and root herbivory on tobacco (Nicotiana tabacum) by the leaf-chewing insect Spodoptera exigua and the root-galling nematode Meloidogyne incognita. Subsequent changes in secondary chemistry (alkaloids and phenolics) were measured in leaves and roots that were orthostichous (vertically aligned) and nonorthostichous (opposite) from the herbivore-damaged tissues. Aboveground caterpillar herbivory elicited stronger secondary chemical responses in orthostichous compared with nonorthostichous plant tissues, although the magnitude of this difference was greater in leaves than roots. However, belowground nematode herbivory did not affect the secondary chemistry of tobacco leaves, despite inducing strong local responses in roots. Thus, plant vascular architecture can mediate the magnitude of systemic induction in roots as well as in leaves, with stronger responses in tissues that are more closely aligned. As a result, herbivores that co-occur on the same sector of plant (both aboveground and belowground) may be more likely to affect one another via induced responses than herbivores that occur on plant tissues sharing fewer resources.





Similar content being viewed by others
References
Allard, H. A. 1942. Some aspects of the phyllotaxy of tobacco. J. Agric. Res. 64:49–55.
Arnold, T. M., and Schultz, J. C. 2002. Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus. Oecologia 130:585–593.
Arnold, T., Appel, H., Patel, V., Stocum, E., Kavalier, A., and Schultz, J. C. 2004. Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink-source model of plant defense. New Phytol. 164:157–164.
Barker, K. R., and Lucas, G. B. 1984. Nematode parasites of tobacco, pp. 213–242, in W. R. Nickle (ed.). Plant and Insect NematodesMarcel Dekker, New York.
Barker, K. R., and Weeks, W. W. 1991. Relationships between soil and levels of Meloidogyne incognita and tobacco yield and quality. J. Nematol. 23:82–90.
Bezemer, T. M., and Van Dam, N. M. 2005. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 20:617–624.
Bezemer, T. M., Wagenaar, R., Van Dam, N. M., Van Der Putten, W. H., and Wäckers, F. L. 2004. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J. Chem. Ecol. 30:53–67.
Bledsoe, T. M., and Orians, C. M. 2006. Vascular pathways constrain 13C accumulation in large root sinks of Lycopersicon esculentum (Solanaceae). Amer. J. Bot. 93:884–890.
Davis, J. M., Gordon, M. P., and Smit, B. A. 1991. Assimilate movement dictates remote sites of wound-induced gene expression in poplar leaves. Proc. Natl. Acad. Sci. U. S. A 88:2393–2396.
Denno, R. F., and McClure, M. S. 1983. Variable Plants and Herbivores in Natural and Managed Systems. Academic, New York.
Ettema, C. H., and Wardle, D. A. 2002. Spatial soil ecology. Trends Ecol. Evol. 17:177–183.
Frost, C. J., Appel, H. M., Carlson, J. E., De Moraes, C. M., Mescher, M. C., and Schultz, J. C. 2007. Within-plant signaling via volatiles overcomes vascular constraints on systemic signaling and primes responses against herbivores. Ecol. Lett. 10:490–498.
Hanounik, S. B., and Osborne, W. W. 1975. Influence of Meloidogyne incognita on the content of amino acids and nicotine in tobacco grown under gnotobiotic conditions. J. Nematol. 7:332–336.
Hanounik, S. B., and Osborne, W. W. 1977. The relationship between population density of Meloidogyne incognita and nicotine content of tobacco. Nematologica 23:147–152.
Hunter, M. D., and Price, P. W. 1992. Playing chutes and ladders—heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73:724–732.
Hussey, R. S., and Barker, K. R. 1973. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Rep. 57:1025–1028.
Jones, C. G., Hopper, R. F., Coleman, J. S., and Krischik, V. A. 1993. Control of systemically induced herbivore resistance by plant vascular architecture. Oecologia 93:452–456.
Jones, H., Martin, R. V., and Porter, H. K. 1959. Translocation of 14carbon in tobacco following assimilation of 14carbon dioxide by a single leaf. Ann. Bot. London 23:493–510.
Kaplan, I., and Denno, R. F. 2007. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol. Lett. 10:977–994.
Kaplan, I., Halitschke, R., Kessler, A., Sardanelli, S., and Denno, R. F. 2008. Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89:392–406.
Karban, R., and Baldwin, I. T. 1997. Induced Responses to Herbivory. The University of Chicago Press, Chicago.
Keinänen, M., Oldham, N. J., and Baldwin, I. T. 2001. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J. Agric. Food Chem. 49:3553–3558.
Mutikainen, P., Walls, M., and Ovaska, J. 1996. Herbivore-induced resistance in Betula pendula: the role of plant vascular architecture. Oecologia 108:723–727.
Orians, C. 2005. Herbivores, vascular pathways, and systemic induction: facts and artifacts. J. Chem. Ecol. 31:2231–2242.
Orians, C. M., Pomerleau, J., and Ricco, R. 2000. Vascular architecture generates fine scale variation in systemic induction of proteinase inhibitors in tomato. J. Chem. Ecol. 26:471–485.
Orians, C. M., Ardon, M., and Mohammad, B. A. 2002. Vascular architecture and patchy nutrient availability generates within-plant heterogeneity in plant traits important to herbivores. Am. J. Bot. 89:270–278.
Orians, C. M., Van Vuuren, M. M. I., Harris, N. L., Babst, B. A., and Ellmore, G. S. 2004. Differential sectoriality in long-distance transport in temperate tree species: evidence from dye flow, 15N transport, and vessel element pitting. Trees 18:501–509.
Rosenberg, M. S., Adams, D. C., and Gurevitch, J. 2000. MetaWin: Statistical Software for Meta-analysis, Version 2.0. Sinauer, Sunderland.
Schittko, U., and Baldwin, I. T. 2003. Constraints to herbivore-induced systemic responses: bidirectional signaling along orthostichies in Nicotiana attenuata. J. Chem. Ecol. 29:763–770.
Shelton, A. L. 2005. Within-plant variation in glucosinolate concentrations of Raphanus sativus across multiple scales. J. Chem. Ecol. 31:1711–1732.
Sokal, R. R., and Rohlf, F. J. 1994. Biometry. Freeman, New York.
Soler, R., Bezemer, T. M., Van Der Putten, W. H., Vet, L. E. M., and Harvey, J. A. 2005. Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J. Anim. Ecol. 74:1121–1130.
Soler, R., Bezemer, T. M., Cortesero, A. M., Van Der Putten, W. H., Vet, L. E. M., and Harvey, J. A. 2007. Impact of foliar herbivory on the development of a root-feeding insect and its parasitoid. Oecologia 152:257–264.
Sprugel, D. G., Hinckley, T. M., and Schaap, W. 1991. The theory and practice of branch autonomy. Annu. Rev. Ecol. Syst. 22:309–334.
Stout, M. J., Workman, K. V., and Duffey, S. S. 1996. Identity, spatial distribution, and variability of induced chemical responses in tomato plants. Entomol. Exp. Appl. 79:255–271.
Trudgill, D. L., and Blok, V. C. 2001. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopath. 39:53–77.
Van Dam, N. M., and Bezemer, T. M. 2006. Chemical communication between roots and shoots: towards an integration of aboveground and belowground induced responses in plants, pp. 127–143, in M. Dicke, and W. Takken (eds.). Chemical Ecology: from Gene to EcosystemSpringer, Dordrecht.
Van Dam, N. M., and Raaijmakers, C. E. 2006. Local and systemic induced responses to cabbage root fly larvae (Delia radicum) in Brassica nigra and B. oleracea. Chemoecology 16:17–24.
Van Dam, N. M., Harvey, J. A., Wäckers, F. L., Bezemer, T. M., Van Der Putten, W. H., and Vet, L. E. M. 2003. Interactions between aboveground and belowground induced responses against phytophages. Basic Appl. Ecol. 4:63–77.
Van Dam, N. M., Raaijmakers, C. E., and Van Der Putten, W. H. 2005. Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol. Exp. Appl. 115:161–170.
Vereecke, D., Messens, E., Klarskov, K., De Bruyn, A., Van Montagu, M., and Goethals, K. 1997. Patterns of phenolic compounds in leafy galls of tobacco. Planta 201:342–348.
Viswanathan, D. V. and Thaler, J. S. 2004. Plant vascular architecture and within-plant spatial patterns in resource quality following herbivory. J. Chem. Ecol. 30:531–543.
Voelckel, C., and Baldwin, I. T. 2004. Generalist and specialist lepidopteran larvae elicit different transcriptional responses in Nicotiana attenuata, which correlate with larval FAC profiles. Ecol. Lett. 7:770–775.
Vovlas, N., Simoes, N. J. O., Sasanelli, N., Dos Santos, M. C. V., and Abrantes, I. M. D. 2004. Host–parasite relationships in tobacco plants infected with a root-knot nematode (Meloidogyne incognita) population from the Azores. Phytoparasitica 32:167–173.
Vuorisalo, T., and Hutchings, M. J. 1996. On plant sectoriality, or how to combine the benefits of autonomy and integration. Vegetatio 127:3–8.
Wardle, D. A. 2002. Communities and Ecosystems: Linking the Aboveground and Belowground Components. Princeton University Press, Princeton.
Wardle, D. A., Bardgett, R. D., Klironomos, J. N., Setälä, H. H., Van Der Putten, W. H., and Wall, D. H. 2004. Ecological linkages between aboveground and belowground biota. Science 304:1629–1633.
Watson, M. A., and Casper, B. B. 1984. Morphogenetic constraints on patterns of carbon distribution in plants. Annu. Rev. Ecol. Syst. 15:233–258.
Wheeler, T. A., Barker, K. R., and Schneider, S. M. 1991. Yield–loss models for tobacco infected with Meloidogyne incognita as affected by soil moisture. J. Nematol. 23:365–371.
Zanne, A. E., Lower, S. S., Cardon, Z. G., and Orians, C. M. 2006. 15N partitioning in tomato: vascular constraints versus tissue demand. Funct. Plant Biol. 33:457–464.
Acknowledgements
Brian Crawford assisted with harvesting plants for secondary metabolite analyses. We thank Jen Thaler for the use of growth chamber space and rhodamine-B in the dye tracer experiment. The chemical characterization was supported by National Science Foundation grant DBI-0500550.
Author information
Authors and Affiliations
Corresponding author
Additional information
Miscellaneous
Robert F. Denno, deceased
Rights and permissions
About this article
Cite this article
Kaplan, I., Halitschke, R., Kessler, A. et al. Effects of Plant Vascular Architecture on Aboveground–Belowground-Induced Responses to Foliar and Root Herbivores on Nicotiana tabacum . J Chem Ecol 34, 1349–1359 (2008). https://doi.org/10.1007/s10886-008-9541-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9541-0