Abstract
Background: To investigate the safety and feasibility of six sessions of Hybrid Assistive Limb (HAL) robot-assisted gait training (RAGT) integrated into an inpatient therapy concept and their influence on walking speed and gait parameters in adult CP patients. Methods: Eleven subjects (male = 8, female = 3, mean age: 23 years and 2 months, ± 4.5 years) with spastic CP underwent six 20-minute RAGT sessions with the HAL during an 11-day hospital stay. Additionally, physiotherapy, physician-performed manual medicine, massage and exercise therapy were provided. Pre- (T1) and post- (T2) intervention assessments were: 10-metre walking test (10MWT), 6-minute walking test (6MWT), Gross Motor Function Measure (GMFM-88) and lower extremities passive range of motion (pROM). Results: All subjects completed the study. No adverse events were noted. Walking speed in the 10MWT test increased from 32.5 s (± 24.5 s) at T1 to 27.5 s (± 21.4 s) at T2, without significance. Slight, but non-significant improvements were detected in the 6MWT, GMFM and pROM. Confounding factors did not significantly affect the results. Conclusion: Intensive therapy including HAL training leads to non-significant improvements. Further studies with more patients and longer intervention time could provide further insights into the RAGT therapy of adult patients with CP. Registration DRKS-ID: DRKS00020275.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral palsy (CP) is one of the most common and most expensive paediatric chronic movement disorders (Oskoui et al., 2013). The estimated lifetime cost per patient with CP in the USA and Europe amounts to as much as $ 921,000 (Shih et al., 2018; Oskoui et al., 2013) report a prevalence of 2.1/1000 live births in their meta-analysis, and in some countries incidences of up to 3.2 cases per 1000 births have been recorded (Johnson, 2002; McGuire et al., 2019). Management of patients with CP includes a detailed assessment of motor skills, provision of positioning and mobility aids and health information (Graham et al., 2016). Activity-based therapy approaches such as physiotherapy are the treatment options which have been most intensively studied and shown most evidence of success (Damiano, 2006; Novak et al., 2013). Since the early 2000s, the range of therapies has been supplemented by various forms of robot-assisted gait training (RAGT) (Rosen and Ferguson, 2020) which provide more contextual and sustainable support to patients and improve functional therapy outcomes.
Since the early 2000s various exoskeletons were developed to guide and support walking (Rodríguez-Fernández et al., 2021). Up to now research on the use of RAGT for patients with CP shows that this method has positive effects on different walking parameters and motor functions, with significant changes in gait speed, mean step length or cadence (Bunge et al., 2021). The Hybrid Assistive Limb (HAL) seems to be the only RAGT using electromyographic (EMG) biofeedback to interact with the patient using it, making the HAL unique. Most of the studies using the HAL reported on single cases or case series with different numbers of interventions (1 to 16 training sessions) and patients’ characteristics. Quality assessed with the critical McMaster appraisal tool showed scores from 36.3 to 72.7% (Bunge et al., 2021). The targeted GMFCS levels range from level I to IV (n = 62) with the majority of subjects classified with level II and III (n = 38), mainly diagnosed with spastic diplegic CP (n = 39) and a mean age from 8.5 to 18.2 years (Bunge et al., 2021). No studies using the HAL specified the subjects daily abilities or need for care. Matsuda et al. (2018a, 2018b), Takahashi et al. (2018) and Ueno et al. (2019) included children but adults with CP as well in their studies. Only Nakagawa et al. (2019) reported in their case study mean Borg Scale values of 11.9 (range 11–13) in twelve sessions of HAL training (n = 1) to determine the subjects physical effort. Studies with other exoskeletons without EMG biofeedback on patients with CP using i.e. the Lokomat only focus on children with CP and do not find any statistically significant changes in outcome variables such as walking speed or step length (Olmos-Gómez et al., 2021).
Studies on adult patients after spinal cord injury have also showed promising results after the use of RAGT regarding functional (Jansen et al., 2017) and neuroplastic aspects (Sczesny-Kaiser et al., 2015). However, although there is some literature showing a positive effect of RAGT on people with CP generally, no study has so far focused specifically on adults with CP.
The aim of the study was to investigate whether RAGT is safe and feasible for adults with CP during an 11-day hospital stay (GMFCS level II and III), and furthermore, if six sessions of HAL gait training integrated into an inpatient therapy concept have a significant influence on walking speed and gait parameters in adults with CP. We hypothesize that RAGT with HAL during a 11-day therapy inpatient stay can be delivered feasible and safe in adult patients with CP. It might change results walking test, such as 10-metre walking test (10MWT), in short term.
Participants and Method
Study Design
This study is a single group, single centre observational study assessing pre- and post-intervention results. The study protocol was approved by the ethics committee of the Westphalian Wilhelms University of Münster, Germany (2019-581-f-S) and was conducted according to the Declaration of Helsinki. The study protocol was explained to the subjects and parents by a physiotherapist and a physician and illustrated by videos (Klinik für Manuelle Therapie (Hamm), 2020a; Klinik für Manuelle Therapie (Hamm)), 2020b). Written informed consent was obtained before the intervention was carried out. The study was conducted in accordance to the Declaration of Helsinki at the Klinik for Manuelle Therapie, Hamm, Germany (KMT).
Recruitment and Blinding
Recruitment consecutively took place in the tetraparesis department of the KMT as a sample of convenience. Due to the availability of the HAL the recruitment was performed from May 2021 to December 2021. The inclusion and exclusion criteria were based on existing literature and the mechanical properties of the HAL. Table 1 gives an overview of the inclusion and exclusion criteria of the study. All participants were recruited from the patient population from the clinic. Blinding of the therapist or participants due to the kind of intervention was not possible. The data were analysed anonymously.
Study Protocol
Participants participated in the study during an inpatient stay of 11 days. The participants received the standard therapy concept of the KMT and, additionally, six sessions of RAGT with the HAL (Fig. 1).
Study design. GMFM-88: Gross Motor Function Measure (88 tasks); Illustration modified from Ammann-Reiffer et al., (2017)
Hybrid Assistive Limb
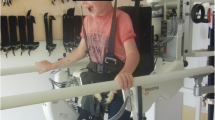
The HAL (lower limb type ML-05, Cybderdyne Inc., Tsukuba, Japan) is a 14 kg rigid exoskeleton enabling individual joint support by electric motors. Electromyogram skin surface electrodes take the lower extremity muscles’ information and the HAL transforms them into robotic joint movement at the level of the knee and hip joints on sagittal plane (Kawamoto & Sankai, 2005). Interactive biofeedback (iBF) is used to graphically display the leg muscles’ derivations and range of motion to the participant and physical therapist. The RAGT took place in an inpatient setting on a treadmill (Kardiomed Mill, Proxomed Medizintechnik GmbH, Alzenau, Germany) using an overhead lift (HM 2815LRC, Handi-Move International, Ninove, Belgium). The HAL was used in cybernetic control mode with individual settings for each participant.
Therapy Concept (Intervention)
The robot-assisted gait training with the HAL was carried out for all participants by one physiotherapist who was specially trained by Cyberdyne Inc. prior to the study performance. For each participant was six sessions of gait training with the HAL each 90 min were planned. Each training session includes the actual walking time in the HAL (20 min), time for putting on and taking off the HAL, rests and evaluation adverse events. In addition to the HAL training during the 11-day inpatient clinic stay, the participants also participated in the clinic’s therapy concept presented in Table 2. The therapy focused primarily on individual movement restrictions, pain modalities and functional abilities with the goal of achieving patient participation.
Outcome Measures
The functional assessments were performed at day 1 (T1) and day 11 (T2) of the 11-day clinic stay. Primary outcome measure was the 10-metre walking test (10MWT) with self-selected walking speed (SSW). Secondary outcome measures were the 10MWT with maximum walking speed (max.). Gross Motor Function Measure (GMFM total and dimensions standing [D], walking, running and jumping [E]), 6-minute walking test (6MWT), Pedoscan and passive range of motion (pROM) of the lower extremities.
Statistical Analysis
Descriptive analyses were done to summarize socio-demographic, clinical and gross motor characteristics. Normal distributions of the outcome variables were evaluated based on visual observation of histograms. We compared baseline values of walking and gross motor parameters by a multivariate analysis of covariances (MANCOVA) with repeated measures “within” factors. Confounding variables were selected based on the socio-demographic, clinical and gross motor characteristics. As the RAGT was added to a standard therapy concept of the KMT, a interim analysis or stopping guidelines were planned. A total of 18 subjects would be sufficient to detect an 0.05 m/s significant increase of walking speed in 10MWT (SSW) with an 80% power. This sample size and power was calculated using the software G*Power (version 3.1, Fraul, Erdfelder, Lang, and Buchner, Kiel, Schleswig-Holstein, Germany). A level of p < 0.05 was used to indicate statistical significance. All data were analysed using the Statistical Package for Social Sciences (SPSS) version 28 (IBM Corp., Armonk, NY, USA). Adverse events were graded according to Common Terminology Criteria for Adverse Events version 5.0. The minimal important difference (MCID) in 10MWT in adults with different diagnoses, such as spinal cord injury, stroke and traumatic brain injury, is described with 0.05–0.14 m/s (Watson, 2002; Perera et al., 2006; Musselman, 2007). MCID in 6MWT range from 34.4 to 50 m in geriatric patients and subjects with stroke (Perera et al., 2006; Tang et al., 2012). Gross Motor Functions Measurement (GMFM-88) is indicated to have a total score MCID of 0.1–3.0% in children with CP (Storm et al., 2020). No data is available concerning adults with CP.
Results
Eleven (male = 8; female = 3) participants were recruited consecutively from the tetraparesis department of the hospital. Mean age of 23 years and 2 months (± 4.5 years, range 18–33 years). Average height was 162.4 cm (± 4.5 cm; range 152–168 cm), mean BMI was 23.9 kg/m2 (± 4.6; range 16.5–32.2 kg/m2). All subjects were classified according to Communication Function Classification System as level I and according to the GMFCS as GMFCS III (n = 10) and GMFCS II (n = 1). Current medication only was baclofen (n = 2). All participants used a wheelchair and various additional aids: 4-point walking stick (n = 2), 1-point walking sticks (n = 1), retro-walker (n = 2), forearm crutches (n = 1), 4-point walking sticks and a retro-walker (n = 4) and 1-point walking sticks and a rollator (n = 1). Table 3 gives summarizes the participants’ characteristics.
Feasibility and Safety
All participants were able to complete the study without any undesired adverse events, such as skin lesions, fatigue, or pain. The HAL could be attached in all subjects easily by a specially trained physical therapist. Attachment of the HAL took 15–20 min depending on the GMFCS level and the individual motor abilities of a participant. Bodyweight support during walking on the treadmill was needed in all participants. In the progress of the RAGT four patients were able to walk free without handrail for a short time. All participants were able to feel the individual support of the HAL during standing and walking. They all made positive comments on the feeling of having no gap between initiation of the movement and support the by the HAL. All participants felt supported and facilitated by using the HAL. As participants with visual limitations, e.g., 13.0 diopters, have severe problems in limb coordination and recognizing the different indicating lines of iBF on the HAL monitor or laptop screen, they expressed that the clear movement input from the HAL is helping them to move more appropriate. Overall, all participants were able to explore coordination and harmonization of lower limb movements on sagittal plane and felt it’s impact on postural alignment during walking and standing.
All subjects performed all six RAGT sessions with the HAL. The average total walking distance in the HAL in all six sessions was 895.3 metres (± 604.1 metres; range 136.7–2054.2 metres). With mixed feelings of motivation and respect using the HAL as a walking aid first time, all participants grow familiar with the HAL in the first two RAGT sessions. The main aspects getting used to the HAL were the participant’ control over the robot support, a calm and safe therapy setting and a good relationship to the serving physical therapist. All participants increased their walking distance in six sessions of RAGT with the HAL in the course of the training sessions.
Evaluation of the data was performed via histograms and showed normal distribution. For the analysis of the functional assessments, a MANCOVA with repeated measurements was calculated with 10MWT (SSW), 10MWT (max), the 6MWT, the GMFM (total) and the GMFM (D + E). There were no significant changes from T1 to T2 across the main effect time within-subjects (F (5,1) = 25.982, p = 0.148; partial η2 = 0.992, n = 11). For the included confounding variables age, gender, weight, height and Barthel Index value in interaction with the main factor time, no significant influence on the change in the results of the above-mentioned functional assessments could be reported either. Table 4 gives an overview of the inner subject contrasts of the dependent variables. Due to the low number of participants the study is underpowered.
The time required in the 10MWT (SSW) changed from 46.0 ± 38.8 s (MW ± SD) (T1) to 35.7 ± 28.1 s (T2) (F = (1,5) 0,006; p = 0,942) (Fig. 2). As covariates age, sex, weight, height and Barthel Index value were included in the statistical model.
Results of the walking tests. 10-metre walking test with SSW (A) and maximum walking speed (B) and results of the 6-minute walking test (C). Boxplots: Middle line: median; lower whisker: minimum value; upper whisker: maximum value; circles: Outliers (1.5 times interquartile range); SSW: Self-Selected Walking Speed; T1: measurement pre-intervention; T2: measurement post-intervention; p < 0.05; Confidence interval: 95%; n = 11.
Results of the Gross Motor Function Measure. GMFM in total (A) and Dimension D + E (B). Boxplots: Middle line: median; lower whisker: minimum value; upper whisker: maximum value; circles: Outliers (1.5 times interquartile range); T1: measurement pre-intervention; T2: measurement post-intervention; p < 0.05; Confidence interval: 95%; n = 11.
Secondary Outcome
10-metre Walking Test (max.)
The time required in the 10MWT (max.) changed from 32.5 ± 24.5 s (T1) to 27.5 ± 21.4 s (T2) (F = (1,5) 0,010; p = 0,925) (Fig. 2). As covariates age, sex, weight, height and Barthel Index value were included in the statistical model.
6-minute Walking Test
The distance walked in the 6MWT increased from 111.1 ± 60.8 m (T1) to 115.5 ± 58.1 m (T2) (F = (1,5) 0,167; p = 0,700) (Fig. 2). As covariates age, sex, weight, height and Barthel Index value were included in the statistical model.
Gross Motor Function Measure
The GMFM (total) scores increased from 64.9 ± 13.5% (T1) to 67.6 ± 12.0% (T2) (F = (1,5) 3,388; p = 0,125). The GMFM (D + E) scores increased from 38.8 ± 16.8% (T1) to 42.4 ± 16.7% (T2) (F = (1,5) 4,954; p = 0,077) (Fig. 3). As covariates age, sex, weight, height and Barthel Index value were included in the statistical model.
Passive Range of Motion of the Lower Extremities
A multivariate analysis of variance was not possible due to insufficient degrees of freedom of the residuals. The results of the passive range of motion of the lower extremities are therefore considered descriptively (Table 5). A slight improvement in the passive range of motion of the measured degrees of freedom except for the mobility of the hip joint in lateral rotation (both sides) was observed. The improvements were not statistically significant.
Discussion
The results of this study show that the use of the HAL in an inpatient setting is feasible and safe. RAGT with the HAL gives adults with CP the possibility to explore their lower limb movement, somatosensory system, and posture without adverse events. Adult participants were motivated to work on their motor performance by using an exoskeleton as walking aid. RAGT with the HAL combined with an additional inpatient therapy concept consisting of physiotherapy, massage, medical exercise therapy and physician-performed manual medicine may have an influence on motor and gait parameters. None of these changes is statistically significant.
The current literature consists of studies with heterogeneous study protocols, a variety of dosage parameters, outcome measures, age ranges and different approaches to statistical analysis not including confounding variables. Thus, comparison of the results is difficult. The special feature of the HAL is that the participant are required to initiate movement themselves, they are not moved by the device only. No studies were found comparing the efficacy or energy expenditure of different types of RAGT or End-effector devices. Most studies examine children with CP (Lefmann et al., 2017). Only Lerner et al. (2017), Ueno et al. (2019) und Orekhov et al. (2020) included people with CP older than 18 years in their research. There has so far been no follow-up evaluation after HAL intervention, and there is no supporting evidence regarding the effects of frequency and duration of sessions or breaks between the sessions.
With the consensus of the motor plateau in people with CP in adolescence (Bottos et al., 2001; Jahnsen et al., 2004; Day et al., 2007; Hanna et al., 2009) point out that the number of subjects with CP who can walk decreases after the age of 18 years. As many as 34% of children who walk unsteadily and sometimes use a wheelchair appear to lose walking function as they age. However, Pirpiris et al., (2006) state that the strongest predictors of satisfaction in 90 children with CP are the ability to stand and perceived pain. Adults with CP experience disadvantages in social life and the labour market (Michelsen et al., 2006). About 40% of adult CP patients have a job only and less than 30% live independently (van Gorp et al., 2020). For adults with CP, it seems to be more important to develop the communication and technical skills required for the workplace so that they can communicate with and control their environment than to focus on small functional improvements (Moll & Cott, 2013). However, besides the mechanical characteristics affecting gait in patients with CP, factors such as cardiovascular endurance, cognitive abilities, and associated impairments such as visual limitations also influence patients’ capabilities (ACPR, 2013; Gage, 2009).
Clinical Relevance
This study suggests the potential of RAGT with HAL for adults with CP to change walking parameters, such as 10MWT. Therefore, patients’ characteristics, training parameters and different neurological conditions and disorders need to be evaluated in further studies to get a clear idea of which group of patients is likely to benefit most from this therapy. Improvements of 10.3 s in the 10MWT (total) and 5 s in the 10MWT (max) seems to be clinically relevant in other populations. Changes in 6MWT results of 4.4 m do not reflect a clinically relevant change. An increase in the total score of the GMFM of 2.7% may present a clinically relevant change depending on the individual achievements. The assessment of the passive range of motion of the lower extremities also revealed positive changes. Since fast and clear motor progress in adults with CP is not to be expected, the time interval between the pre- and post-treatment assessments can be regarded as very short.
The use of the HAL is motivative and the activity required of the patient is an advantage for other devices. Because of interactive biofeedback, it is not possible for a patient to just consume the HAL therapy and pretend to be involved actively. Due to the high cost of RAGT, this form of therapy is available to very few patients. Independent use of the HAL at home is not possible. More standards need to be created under which patient-related and economic aspects of RAGT can be implemented. Much more research should also be done on the impact of the cardiovascular aspects of RAGT-supported exercise on adults with CP and their comorbidities.
From a qualitative point of view, it should be emphasized that RAGT supports participant’ movement not only passively, but also clearly assists and completes possible movements which are actively initiated by the participants. All participants felt motivated by the HAL to move more and enjoyed being part of this kind of robot-assisted therapy. The use of the HAL in patients with CP has many benefits such as: (i) creating a safe and motivating environment to explore walking abilities, (ii) supporting power and (iii) range of motion in highly repetitive walking training and increasing the safety in pre-walking motor abilities such as standing.
Limitations
The present study has limitations. Due to the low sample size, the study is underpowered. Because the availability of the HAL was only temporary and patients’ concerns about COVID-19 infections, it was not possible to include more subjects in the study. The statistical model had to be adjusted and covariates had to be manually removed from the statistical model because of the insufficient degrees of freedom of the residuals. Additionally, no follow-up data were collected and therefore no medium- or long-term effect can be reported.
Due to the parallel application of RAGT sessions with physiotherapy, physician performed manual medicine, massage and exercise therapy, a clear performance bias must be pointed out. However, a control group without standard therapy or intervention could not be implemented for ethical reasons.
Conclusion
The results indicate that RAGT with HAL can be performed safely in a hospital setting with adults with CP. Adults with CP show functional improvements after intensive active therapy with the HAL embedded in a participation-orientated therapy concept. However, these changes are not significant. The treatment approach with the HAL may be promising and needs to be evaluated under adjusted conditions such as larger sample sizes, longer intervention time for the single session as well for the intervention period. Much more qualitative research could provide further insights into the subjects’ progress and clinical outcomes. Further studies need to be conducted to establish a patient profile of the patient groups that benefit most from RAGT and specific training parameters.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- 10MWT:
-
10-metre Walking Test
- 6MWT:
-
6-minute Walking Test
- AFO:
-
Ankle-Foot Orthosis
- AO:
-
Ankle Orthosis
- GMFCS:
-
Gross Motor Function Classifications System
- GMFM-88:
-
Gross Motor Functions Measure (88 tasks)
- HAL:
-
Hybrid Assistive Limb
- iBF:
-
interactive Biofeedback
- KMT:
-
Klinik für Manuelle Therapie
- MANCOVA:
-
Multivariate Analysis of Covariance
- pROM:
-
Passive Range of Motion
- RAGT:
-
Robot-assisted gait training
- SSW:
-
Self-selected walking speed
- T1:
-
Day 1 (pre intervention assessment)
- T2:
-
Day 11 (post intervention assessment)
References
ACPR (2013). Australian Cerebral Palsy Register (ACPR) Report 2013 (S. 64). https://cerebralpalsy.org.au/wp-content/uploads/2013/04/ACPR-Report_Web_2013.pdf
Ammann-Reiffer, C., Bastiaenen, C. H. G., Meyer-Heim, A. D., & van Hedel, H. J. A. (2017). Effectiveness of robot-assisted gait training in children with cerebral palsy: a bicenter, pragmatic, randomized, cross-over trial (PeLoGAIT). BMC Pediatrics, 17(1), 64. https://doi.org/10.1186/s12887-017-0815-y.
Bottos, M., Feliciangeli, A., Sciuto, L., Gericke, C., & Vianello, A. (2001). Functional status of adults with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. ;43:516–528. doi: https://doi.org/10.1017/s0012162201000950. PMID: 11508917.
Bunge, L. R., Davidson, A. J., Helmore, B. R., Mavrandonis, A. D., Page, T. D., Schuster-Bayly, T. R., & Kumar, S. (2021). Effectiveness of powered exoskeleton use on gait in individuals with cerebral palsy: a systematic review. PLOS ONE, 16(5), e0252193. https://doi.org/10.1371/journal.pone.0252193.
Damiano, D. L. (2006). Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. ;86:1534–1540. doi: https://doi.org/10.2522/ptj.20050397. PMID: 17094192.
Day, S. M., Wu, Y. W., Strauss, D. J., Shavelle, R. M., & Reynolds, R. J. (2007). Change in ambulatory ability of adolescents and young adults with cerebral palsy. Dev Med Child Neurol. ;49:647–653. doi: https://doi.org/10.1111/j.1469-8749.2007.00647.x. PMID: 17718819.
Gage, J. R. (2009). Gait Pathology in individuals with cerebral palsy. In J. R. Gage, M. H. Schwartz, & S. E. Koop(Hrsg.) The identification and treatment of gait problems in cerebral palsy (pp. 65–177). Mac Keith Press.
Graham, H. K., Rosenbaum, P., Paneth, N., Dan, B., Lin, J. P., Damiano, D. L., Becher, J. G., Gaebler-Spira, D., Colver, A., Reddihough, D. S., Crompton, K. E., & Lieber, R. L. (2016). Cerebral palsy. Nature Reviews Disease Primers, 2(1), 15082. https://doi.org/10.1038/nrdp.2015.82.
Hanna, S. E., Rosenbaum, P. L., Bartlett, D. J., Palisano, R. J., Walter, S. D., Avery, L., & Russell, D. J. (2009). Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Developmental Medicine & Child Neurology, 51(4), 295–302. https://doi.org/10.1111/j.1469-8749.2008.03196.x.
Jahnsen, R., Villien, L., Egeland, T., Stanghelle, J. K., & Holm, I. (2004). Locomotion skills in adults with cerebral palsy. Clin Rehabil. ;18:309–316. doi: https://doi.org/10.1191/0269215504cr735oa. PMID: 15137562.
Jansen, O., Schildhauer, T. A., Meindl, R. C., Tegenthoff, M., Schwenkreis, P., Sczesny-Kaiser, M., Grasmücke, D., Fisahn, C., & Aach, M. (2017). Functional Outcome of Neurologic-Controlled HAL-Exoskeletal Neurorehabilitation in Chronic Spinal Cord Injury: A Pilot With One Year Treatment and Variable Treatment Frequency. Global Spine J. ;7:735–743. doi: https://doi.org/10.1177/2192568217713754. PMID: 29238636.
Johnson, A. (2002). Prevalence and characteristics of children with cerebral palsy in Europe. Developmental Medicine & Child Neurology, 44(09), https://doi.org/10.1017/S0012162201002675.
Kawamoto, H., & Sankai, Y. (2005). Power assist method based on phase sequence and muscle force condition for HAL. Advanced Robotics, 19(7), 717–734. https://doi.org/10.1163/1568553054455103.
Klinik für Manuelle Therapie (Hamm) (Regisseur) (2020a). Erfolgreiche Anwendung des HAL-Systems bei Kindern. https://youtu.be/xE3AMKz_dWw. Accessed 27 May 2022
Klinik für Manuelle Therapie (Hamm)) (Regisseur) (2020b). Klinische Studie an der KMT Hamm. https://youtu.be/yOsAS93xg0A. Accessed 27 May 2022
Lefmann, S., Russo, R., & Hillier, S. (2017). The effectiveness of robotic-assisted gait training for paediatric gait disorders: systematic review. Journal of NeuroEngineering and Rehabilitation, 14(1), 1. https://doi.org/10.1186/s12984-016-0214-x.
Lerner, Z. F., Damiano, D. L., & Bulea, T. C. (2017). A lower-extremity exoskeleton improves knee extension in children with crouch gait from cerebral palsy. Sci Transl Med. ;9:eaam9145. doi: https://doi.org/10.1126/scitranslmed.aam9145. PMID: 28835518.
Matsuda, M., Iwasaki, N., Mataki, Y., Mutsuzaki, H., Yoshikawa, K., Takahashi, K., Enomoto, K., Sano, K., Kubota, A., Nakayama, T., Nakayama, J., Ohguro, H., Mizukami, M., & Tomita, K. (2018b). Robot-assisted training using hybrid assistive Limb® for cerebral palsy. Brain and Development, 40(8), 642–648. https://doi.org/10.1016/j.braindev.2018.04.004.
Matsuda, M., Mataki, Y., Mutsuzaki, H., Yoshikawa, K., Takahashi, K., Enomoto, K., Sano, K., Mizukami, M., Tomita, K., Ohguro, H., & Iwasaki, N. (2018a). Immediate effects of a single session of robot-assisted gait training using Hybrid Assistive Limb (HAL) for cerebral palsy. Journal of Physical Therapy Science, 30(2), 207–212. https://doi.org/10.1589/jpts.30.207.
McGuire, D. O., Tian, L. H., Yeargin-Allsopp, M., Dowling, N. F., & Christensen, D. L. (2019). Prevalence of cerebral palsy, intellectual disability, hearing loss, and blindness, National Health interview Survey, 2009–2016. Disability and Health Journal, 12(3), 443–451. https://doi.org/10.1016/j.dhjo.2019.01.005.
Michelsen, S. I., Uldall, P., Hansen, T., & Madsen, M. (2006). Social integration of adults with cerebral palsy. Dev Med Child Neurol. ;48:643–649. doi: 10.1017/S0012162206001368. PMID: 16836775.
Moll, L. R., & Cott, C. A. (2013). The paradox of normalization through rehabilitation: growing up and growing older with cerebral palsy. Disability and Rehabilitation, 35(15), 1276–1283. https://doi.org/10.3109/09638288.2012.726689.
Musselman, K. E. (2007). Clinical significance testing in rehabilitation research: what, why, and how? Physical Therapy Reviews, 12(4), 287–296. https://doi.org/10.1179/108331907X223128.
Nakagawa, S., Mutsuzaki, H., Mataki, Y., Endo, Y., Kamada, H., & Yamazaki, M. (2019). Improvement and sustainability of walking ability with hybrid assistive limb training in a patient with cerebral palsy after puberty: a case report. Journal of Physical Therapy Science, 31(8), 633–637. https://doi.org/10.1589/jpts.31.633.
Novak, I., McIntyre, S., Morgan, C., Campbell, L., Dark, L., Morton, N., Stumbles, E., Wilson, S. A., & Goldsmith, S. (2013). A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. ;55:885–910. doi: https://doi.org/10.1111/dmcn.12246. PMID: 23962350.
Olmos-Gómez, R., Gómez-Conesa, A., Calvo-Muñoz, I., & López-López, J. A. (2021). Effects of robotic-assisted gait training in children and adolescents with cerebral palsy: a Network Meta-Analysis. Journal of Clinical Medicine, 10(21), 4908. https://doi.org/10.3390/jcm10214908.
Orekhov, G., Fang, Y., Luque, J., & Lerner, Z. F. (2020). Ankle Exoskeleton Assistance Can Improve Over-Ground Walking Economy in Individuals With Cerebral Palsy. IEEE Trans Neural Syst Rehabil Eng. ;28:461–467. doi: https://doi.org/10.1109/TNSRE.2020.2965029. PMID: 31940542.
Oskoui, M., Coutinho, F., Dykeman, J., Jetté, N., & Pringsheim, T. (2013). An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. ;55:509–519. doi: https://doi.org/10.1111/dmcn.12080. PMID: 23346889.
Perera, S., Mody, S. H., Woodman, R. C., & Studenski, S. A. (2006). Meaningful change and responsiveness in common physical performance measures in older adults: MEANINGFUL CHANGE AND PERFORMANCE. Journal of the American Geriatrics Society, 54(5), 743–749. https://doi.org/10.1111/j.1532-5415.2006.00701.x.
Pirpiris, M., Gates, P. E., McCarthy, J. J., D’Astous, J., Tylkowksi, C., Sanders, J. O., Dorey, F. J., Ostendorff, S., Robles, G., Caron, C., & Otsuka, N. Y. (2006). Function and well-being in Ambulatory Children with cerebral palsy. Journal of Pediatric Orthopaedics, 26(1), 119–124. https://doi.org/10.1097/01.bpo.0000191553.26574.27.
Rodríguez-Fernández, A., Lobo-Prat, J., & Font-Llagunes, J. M. (2021). Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. Journal of Neuroengineering and Rehabilitation, 18(1), 22. https://doi.org/10.1186/s12984-021-00815-5.
Rosen, J., & Ferguson, P. W. (Hrsg.) (2020). Wearable Robotics. Elsevier. https://doi.org/10.1016/C2017-0-01139-4
Sczesny-Kaiser, M., Höffken, O., Aach, M., Cruciger, O., Grasmücke, D., Meindl, R., Schildhauer, T. A., Schwenkreis, P., & Tegenthoff, M. (2015). HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. Journal of NeuroEngineering and Rehabilitation, 12(1), 68. https://doi.org/10.1186/s12984-015-0058-9.
Shih, S. T. F., Tonmukayakul, U., Imms, C., Reddihough, D., Graham, H. K., Cox, L., & Carter, R. (2018). Economic evaluation and cost of interventions for cerebral palsy: a systematic review. Dev Med Child Neurol. ;60:543–558. doi: https://doi.org/10.1111/dmcn.13653. PMID: 29319155.
Storm, F. A., Petrarca, M., Beretta, E., Strazzer, S., Piccinini, L., Maghini, C., Panzeri, D., Corbetta, C., Morganti, R., Reni, G., Castelli, E., Frascarelli, F., Colazza, A., Cordone, G., & Biffi, E. (2020). Minimum Clinically Important Difference of Gross Motor Function and Gait Endurance in Children with Motor Impairment: A Comparison of Distribution-Based Approaches. BioMed Research International, 2020, 1–9. https://doi.org/10.1155/2020/2794036
Takahashi, K., Mutsuzaki, H., Mataki, Y., Yoshikawa, K., Matsuda, M., Enomoto, K., Sano, K., Kubota, A., Mizukami, M., Iwasaki, N., & Yamazaki, M. (2018). Safety and immediate effect of gait training using a hybrid assistive limb in patients with cerebral palsy. Journal of Physical Therapy Science, 30(8), 1009–1013. https://doi.org/10.1589/jpts.30.1009.
Tang, A., Eng, J. J., & Rand, D. (2012). Relationship between perceived and measured changes in walking after stroke. Journal of Neurologic Physical Therapy, 36(3), 115–121. https://doi.org/10.1097/NPT.0b013e318262dbd0.
Ueno, T., Watanabe, H., Kawamoto, H., Shimizu, Y., Endo, A., Shimizu, T., Ishikawa, K., Kadone, H., Ohto, T., Kamada, H. (2019). Feasibility and safety of Robot Suit HAL treatment for adolescents and adults with cerebral palsy. J Clin Neurosci. ;68:101–104. doi: https://doi.org/10.1016/j.jocn.2019.07.026. PMID: 31337581.
van Gorp, M., Hilberink, S. R., Noten, S., Benner, J. L., Stam, H. J., van der Slot, W. M. A., & Roebroeck, M. E. (2020). Epidemiology of Cerebral Palsy in Adulthood: A Systematic Review and Meta-analysis of the Most Frequently Studied Outcomes. Arch Phys Med Rehabil. ;101:1041–1052. doi: https://doi.org/10.1016/j.apmr.2020.01.009. PMID: 32059945.
Watson, M. J. (2002). Refining the ten-metre walking test for Use with neurologically impaired people. Physiotherapy, 88(7), 386–397. https://doi.org/10.1016/S0031-9406(05)61264-3.
Acknowledgements
The authors would like to thank the Heinz and Anneliese Medaljon-Foundation for independent funding of the study. We also thank all the subjects who participated in and supported the study. We would also like to thank the supporting nursing staff and the central patient planning office of the KMT Hamm. We acknowledge Kaye Schreyer for editing the manuscript and also the support by the Open Access Publication Fund of the University of Duisburg-Essen, Germany. We thank Björn Walther for his feedback concerning data evaluation.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was founded by the Heinz und Anneliese Medaljon-Foundation (legal entity identifier LEI# 96760098311CG4ZZ4T46, Funding number: 133/19). The APC was funded by the Open Access Publication Fund of the University of Duisburg-Essen, Germany.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.A., F.M., A.K., A.B. and J.S.; methodology, J.A., F.M., A.K., A.B. and J.S.; data curation, F.M. and A.B.; writing—original draft preparation, F.M.; writing— review and editing, J.A., M.H., M.D., A.K., A.B. and J.S.; visualization, F.M.; supervision, J.A.; project administration, F.M.; funding acquisition, J.A.; F.M and A.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Westphalian Wilhelms University Münster, Germany (2019-581-f-S). Trial Registration, DRKS-ID: DRKS00020275.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moll, F., Kessel, A., Bonetto, A. et al. Safety and Feasibility of Robot-assisted Gait Training in Adults with Cerebral Palsy in an Inpatient Setting – an Observational Study. J Dev Phys Disabil 35, 1091–1106 (2023). https://doi.org/10.1007/s10882-023-09895-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10882-023-09895-8