Abstract
AnaConDa-100 ml (ACD-100, Sedana Medical, Uppsala, Sweden) is well established for inhalation sedation in the intensive care unit. But because of its large dead space, the system can retain carbon dioxide (CO2) and increase ventilatory demands. We therefore evaluated whether AnaConDa-50 ml (ACD-50), a device with half the internal volume, reduces CO2 retention and ventilatory demands during sedation of invasively ventilated, critically ill patients. Ten patients participated in this cross-over protocol. After sedation with isoflurane via ACD-100 for 24 h, the 5-h observation period started. During the first hour, ACD-100 was used; for the next 2 h, ACD-50; and for the last 2 h, ACD-100 was used again. Sedation was titrated to Richmond Agitation and Sedation Scale (RASS) score − 3 to − 4 and a processed electroencephalogram (Narcotrend Index, Narcotrend-Gruppe, Hannover, Germany) was recorded. Minute ventilation, CO2 elimination, and isoflurane consumption were compared. All patients were deeply sedated (Narcotrend Index, mean ± SD: 38 ± 10; RASS scores − 3 to − 5) and breathed spontaneously with pressure support throughout the observation period. Infusion rates of isoflurane and opioid, either remifentanil or sufentanil, as well as ventilator settings were unchanged. Minute ventilation and end-tidal CO2 were significantly reduced with the ACD-50, respiratory rate remained unchanged, and tidal volume decreased by 66 ± 43 ml. End-tidal isoflurane concentrations were also slightly reduced while haemodynamic measures remained constant. The ACD-50 reduces the tidal volume needed to eliminate carbon dioxide without augmenting isoflurane consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Volatile anaesthetics are increasingly used for critical care sedation in Europe [1,2,3] and Canada [4]. Since 2013, volatile anaesthetics have been included as acceptable types of sedation in the Spanish, British, and German guidelines [5,6,7].
A well-established method for administering volatile anaesthetics in conjunction with common intensive care ventilators is the anaesthetic conserving device (ACD, AnaConDa®, Sedana Medical, Uppsala, Sweden, Fig. 1). As with heat-and-moisture exchanging filters, the device is inserted between the Y-piece and the patient. Liquid volatile anaesthetic is delivered by a syringe pump into an evaporator incorporated within the ACD. The key element of the ACD is a reflector made of activated carbon that limits loss of the anaesthetic into the open ventilator system [8]. The ACD reflects up to 90% of the exhaled anaesthetic [9].
a AnaConDa-50 ml, b AnaConDa-100 ml, (Sedana Medical, Uppsala, Sweden), (1) ventilator side with Y piece (2) anaesthetic reflector (invisible inside the black case), (3) evaporator (white porous hollow rod inside the transparent case) (4) gas sampling port with sampling line to the gas monitor, (5) patient side of the device, (6) agent supply line for infusion of liquid anaesthetic, c ACD-50: AnaConDa-50 ml, ACD-100: AnaConDa-100 ml
The ACD affects carbon dioxide removal in two manners: first, by its volume of 100 ml (“volumetric” dead space, analogous to the effect of HME filters), secondly, by reflecting exhaled carbon dioxide back to the patient during the next inspiration (“apparent” or “reflective” dead space) [10, 11]. Carbon dioxide retention limits utility of the ACD-100 in patients who require small tidal volumes, including children [12] or patients in acute respiratory distress syndrome (ARDS) [13].
Recently, a new version of the ACD was developed with 50 ml internal volume (ACD-50) instead of 100 ml (ACD-100). The expected consequence is improved carbon dioxide elimination which presumably reduces ventilatory demand. But the small ACD-50 inevitably contains less activated carbon than the 100-ml version which might impair isoflurane reflection. We therefore compared minute ventilation, carbon dioxide elimination, and isoflurane consumption in spontaneously breathing patients sedated with ACD-100 and ACD-50 devices. We hypothesized that minute ventilation decreases.
2 Methods
This retrospective analysis was approved by the Institutional Review Board (Registration number: 170/17, Saarland Medical Chamber, Faktoreistr. 4, 66111 Saarbruecken, Germany). Written informed consent of the patients was obtained after they awakened.
Ten consecutive critically ill patients requiring deep sedation were evaluated. After priming ACD-100 with 1.2 ml isoflurane, the syringe pump (Perfusor; Braun Melsungen AG, Germany) was started at a rate of 3 ml/h and then adjusted, targeting at Richmond Agitation-Sedation Scale (RASS) scores between − 3 and − 4 [14]. Analgesia was provided by remifentanil or sufentanil infusions.
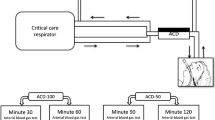
As shown in Fig. 2a, ventilation hoses, Y-piece, the AnaConDa, a tube elongation and the endotracheal tube were connected in-line. Patients were ventilated with an Evita 4 ventilator (Dräger Medical) and received closed endotracheal suctioning. Isoflurane and end-tidal carbon dioxide concentrations were monitored with a Vamos gas monitor (Dräger Medical, Lübeck, Germany) which was connected to the ACD via a sample line. The gas outlet of both the ventilator and the gas monitor was injected into a FlurAbsorb anaesthetic gas filter (Sedana Medical). A processed electroencephalogram (Narcotrend Index, Narcotrend-Gruppe, Hannover, Germany) was recorded.
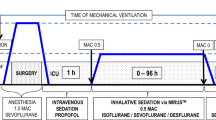
a Setup of the AnaConDa system: ventilation hoses, the AnaConDa (ACD), Y-piece, a tube elongation and the endotracheal tube are connected in line. Liquid isoflurane is supplied via a syringe pump. A processed electroencephalogram (EEG, Narcotrend Index, Narcotrend-Gruppe, Hannover, Germany) was recorded. b Treatment protocol
Isoflurane sedation with the ACD-100 was maintained for 24 h before the 5-h observation period started (Fig. 2b). After 1 h of observation, the endotracheal tube was clamped and the ACD-100 switched for a new ACD-50. The new system was primed with 0.9 ml isoflurane and measurements were continued for 2 h. The tube was clamped again, a new ACD-100 was connected, primed with 1.2 ml isoflurane and measurements were continued for another 2 h. Isoflurane and opioid infusion rates were initially kept constant after each device change, but could subsequently be changed if clinically indicated. When the 5-h protocol ended, the clinical treatment team answered a questionnaire (Supplement 1) about feasibility, safety, and handling—and selected one of the ACD options for continued care.
During the observation period, ventilation and treatment characteristics, the isoflurane infusion rate, RASS scores and Narcotrend indices were documented every 15 min. Arterial blood was sampled for carbon dioxide and oxygen partial pressure analyses every 60 min throughout the 5-h protocol period. Carbon dioxide and isoflurane concentrations were measured and electronically recorded every 100 ms. Patient characteristics, diagnoses, and vital parameters were extracted from the ICU patient management system (Copra, Version 5, Copra System GmbH, Berlin, Germany).
2.1 Data analysis
Data recorded at 15-min intervals were averaged over each study hour and designated ACD-100 (baseline), ACD-50-1 h, ACD-50-2 h, ACD-100-1 h, and ACD-100-2 h. End-tidal carbon dioxide and isoflurane concentrations were extracted from online breath-by-breath recordings and also averaged over each study hour. Isoflurane concentrations were expressed as fractions of the age-adjusted minimal alveolar concentration (MAC) [15]. To account for potential parameter drifts over time, the values of ACD-100 (baseline) and ACD-100-2 h were averaged (ACD-100mean), and statistically compared to those of ACD-50-2 h.
We examined the influence of the isoflurane gas quantity contained in an exhaled breath on the isoflurane reflection of both devices. Specifically, we approximated the isoflurane vapour volume by multiplying expired isoflurane concentrations and tidal volumes of ACD-100 (baseline) and compared this with the difference in end-tidal isoflurane concentrations between both reflectors (ACD-100mean–ACD-50-2 h).
Continuous variables are expressed as means ± standard deviations. Categorical variables are presented as numbers of patients with percentages in parentheses. The statistical significance of changes of continuous variables in each group was tested with ANOVA for repeated measurements and with Bonferroni corrections for multiple testing. ANOVA for nonparametric values (Friedman test) was used for data that were not normally distributed, followed by a multiple comparison method (Bonferroni method). Statistical significance was accepted at two-sided significance level of 0.05. All data analyses were performed using SigmaPlot 12.0 (Jandel Scientific Corp., San Rafael, USA).
3 Results
Ten consecutive patients completed the full 5-h protocol. The patients’ characteristics are reported in Table 1. The sequential organ failure assessment scores were 11.5 ± 4.2 (mean ± standard deviation) on admission.
RASS scores and Narcotrend indices indicated that all patients were deeply sedated throughout the observation period and remained unaffected by switching devices (Table 2). Despite profound sedation, all patients were breathing spontaneously through endotracheal tubes with pressure support and coughed in response to endotracheal suctioning. Isoflurane infusion rates varied among patients, but remained unchanged during the observation period at a rate of 3.1 ± 2.0 ml/h; opioid infusion rates also remained unchanged (Table 2).
The end-tidal concentrations of isoflurane ranged from 0.2 to 0.8 MAC and were slightly greater with the ACD-100 than with the ACD-50 (ACD-100mean versus ACD-50-2 h: 0.55 ± 0.18 versus 0.52 ± 0.19 MAC, p = 0.015, Fig. 3a). Only in two patients the endtidal isoflurane concentration decreased by more than 0.1 MAC (0.16 and 0.11 MAC). Interestingly, these two patients exhaled the highest isoflurane vapour volume, approximately 4.9 and 6.3 ml per breath.
a End-tidal isoflurane concentration; b minute volume; c end-tidal carbon dioxide concentration; before, during, and after ACD-50 use in ten patients. ACD-50 = AnaConDa-50 ml (green). ACD-100 = AnaConDa-100 ml (blue), h hour. Recorded data were averaged over each study hour and designated ACD-100-24 h (baseline), ACD-50-1 h, ACD-50-2 h, ACD-100-1 h, and ACD-100-2 h. Box (first and third quartile) and whiskers (5th and 95th percentiles) plot. Solid dash: median values. Plus symbol: mean values. Points: Outliers (> two standard deviations). Blue box: ACD-100. Green box: ACD-50. *p < 0.05 versus ACD-100mean (average of ACD-100-24 h (baseline) and ACD-100-2 h)
Minute ventilation and end-tidal carbon dioxide were significantly reduced with the ACD-50 (Fig. 3b, c), respiratory rate remained unchanged, and tidal volume decreased by 66 ± 43 ml. Also, peak flows were significantly higher with the ACD-100. Positive end-expiratory pressures and pressure support were not changed by the treatment team. Blood gas analyses showed no difference in arterial pressures of carbon dioxide. Mean arterial pressure, heart rate, and norepinephrine dose did not change significantly during the observation period (Table 2).
No complications or handling problems were observed during the observation period. In the questionnaire, four of ten treatment teams reported that handling the ACD-50 was easier than the ACD-100; the other six reported no remarkable difference in handling. Some appreciated the lighter weight of ACD-50 (38 versus 50 g) which pulled less on the endotracheal tube. 9 of the 10 teams preferred the ACD-50 and thus continued sedation with that device (Table 3).
4 Discussion
In this quality improvement project, we found that ACD-50 reduced tidal volumes and end-tidal carbon dioxide concentrations compared to the older ACD-100 in ventilated patients who breathed spontaneously with constant pressure support. Respiratory rate, hemodynamic variables, and sedation depth, as well as opioid and isoflurane infusion rates remained unchanged.
Previous studies document that the anaesthetic conserving device reflects both volatile anaesthetics and exhaled carbon dioxide molecules. We consider the additional tidal volume that is needed to expel reflected carbon dioxide to be reflective dead space [10]. Reflective dead space was found to be 180–200 ml under dry laboratory conditions [10, 16], 60–80 ml under body temperature pressure conditions [10, 11], and 35 to 46 ml when adding isoflurane [10], or sevoflurane [17]. Combined with the reflector’s internal volume (volumetric dead space), the ACD increases minute ventilation and work of breathing [18]. As might be expected, the smaller ACD-50 significantly diminished the minute ventilation required to maintain constant partial pressure of arterial carbon dioxide. Specifically, spontaneously breathing patients reduced their tidal volumes by 66 ml, corresponding to 50 ml reduced volumetric and 16 ml reduced reflective dead space. In a recent bench study, reflective dead space has been quantified as 40 ml with the ACD-100 and 25 ml with the ACD-50 under clinically relevant conditions, for a difference of 15 ml [19].
There are several reports about inhalation sedation with the ACD in patients with severe acute respiratory distress syndrome, undergoing kinetic lateral rotational therapy [20] or extracorporeal membrane oxygenation [21]. Advantages included stable spontaneous ventilation despite deep sedation [20, 21], and improved oxygenation as well as reduced inflammation [13]. However, increased dead space ventilation was criticized for interfering with lung protective ventilation [17]. Availability of an anaesthetic administration device with less dead space will facilitate inhalational sedation in these and other patients who require small tidal volumes.
There was a small but significant decrease in end-tidal isoflurane concentration when the ACD-100 was switched to the ACD-50 with constant anaesthetic infusion. This result is consistent with a previous bench study [19]. As no changes in RASS scores or Narcotrend indices were observed, the slightly reduced reflection capacity of ACD-50 is not clinically important at low isoflurane concentrations (0.2–0.8 MAC), commonly used for inhalational sedation in the ICU. It was not until exhaled isoflurane reached about 5 ml per breath, corresponding to 1 vol% isoflurane in 500 ml tidal volume, or 0.8 vol% in 625 ml, that the ACD-50s reflection efficiency started to decrease. This is consistent with the concept of the reflection capacity, quantified as the vapour volume expired in one breath, described previously. Once the reflection capacity is exceeded, efficiency of the reflector will deteriorate [9]. The reduced reflection capacity of the smaller device might be important when tidal volumes are large, high isoflurane concentrations are needed, or when less potent volatile anaesthetics such as sevoflurane are used. However, the importance of each factor requires additional study.
No complications were observed consequent to use of the ACD-50. After the observation period, nine of ten treatment teams continued with ACD-50 appreciating the lighter weight and less pulling on the endotracheal tube.
It is a limitation that this was a quality improvement project and not a randomized controlled trial. By using a cross over design with intra-individual comparisons, significant differences could be demonstrated using a small number of patients. We acknowledge that this limited number cannot be representative for all ICU patients, so we cannot infer to children, small adults, or those with very small tidal volumes on the one hand, as far as airway resistance and carbon dioxide retention are concerned, but neither on very large patients with high tidal volumes, as far as anaesthetic reflection capacity is concerned.
We did not measure resistance or specifically quantify work of breathing. But there were no increases in respiratory rate, heart rate, or carbon dioxide concentration, nor were there any clinical signs of dyspnoea, all of which suggest that the work of breathing was not increased by clinically important amounts. Consistent with this theory, ventilation and sedation were well maintained with the ACD-50.
In this quality improvement project with a limited number of patients, we show that reduced dead space of the ACD-50 lowered tidal volumes in spontaneously breathing patients. The ACD-50 reflects sufficient isoflurane to provide profound sedation. However, further evaluation will be necessary, especially in patients requiring ventilation with either extremely low or high tidal volumes. Furthermore, the ACD-50s effect on work of breathing and its combination with other volatile anaesthetics, such as sevoflurane, need to be examined in more detail.
References
Mesnil M, Capdevila X, Bringuier S, Trine PO, Falquet Y, Charbit J, Roustan JP, Chanques G, Jaber S. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Medicine. 2011;37(6):933–41. https://doi.org/10.1007/s00134-011-2187-3.
Romagnoli S, Chelazzi C, Villa G, Zagli G, Benvenuti F, Mancinelli P, Arcangeli G, Dugheri S, Bonari A, Tofani L, Belardinelli A, De Gaudio AR. The new MIRUS system for short-term sedation in postsurgical ICU patients. Crit Care Med. 2017. https://doi.org/10.1097/ccm.0000000000002465.
Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the anesthetic conserving device. Crit Care Med. 2004;32(11):2241–6.
Jerath A, Beattie SW, Chandy T, Karski J, Djaiani G, Rao V, Yau T, Wasowicz M. Volatile-based short-term sedation in cardiac surgical patients: a prospective randomized controlled trial. Crit Care Med. 2015;43(5):1062–9. https://doi.org/10.1097/ccm.0000000000000938.
Baron R, Binder A, Biniek R, Braune S, Buerkle H, Dall P, Demirakca S, Eckardt R, Eggers V, Eichler I, Fietze I, Freys S, Frund A, Garten L, Gohrbandt B, Harth I, Hartl W, Heppner HJ, Horter J, Huth R, Janssens U, Jungk C, Kaeuper KM, Kessler P, Kleinschmidt S, Kochanek M, Kumpf M, Meiser A, Mueller A, Orth M, Putensen C, Roth B, Schaefer M, Schaefers R, Schellongowski P, Schindler M, Schmitt R, Scholz J, Schroeder S, Schwarzmann G, Spies C, Stingele R, Tonner P, Trieschmann U, Tryba M, Wappler F, Waydhas C, Weiss B, Weisshaar G. (2015) Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. GMS German Med Sci. https://doi.org/10.3205/000223.
Grounds M, Snelson C, Whitehouse T, Willson J, Tulloch L, Linhartova L, Shah A, Pierson R, England K. (2014) Intensive care society review of best practice for analgesia and sedation in the critical care. Sedation committee of the Intensive Care Society United Kingdom. http://www.ics.ac.uk/ICS/guidelines-and-standards.aspx. Accessed April 21, 2017.
Celis-Rodriguez E, Birchenall C, de la Cal MA, Castorena Arellano G, Hernandez A, Ceraso D, Diaz Cortes JC, Duenas Castell C, Jimenez EJ, Meza JC, Munoz Martinez T, Sosa Garcia JO, Pacheco Tovar C, Palizas F, Pardo Oviedo JM, Pinilla DI, Raffan-Sanabria F, Raimondi N, Righy Shinotsuka C, Suarez M, Ugarte S, Rubiano S. Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Med Intensiva. 2013;37(8):519–74. https://doi.org/10.1016/j.medin.2013.04.001.
Meiser A, Laubenthal H. Inhalational anaesthetics in the ICU: theory and practice of inhalational sedation in the ICU, economics, risk-benefit. Best Pract Res Clin Anaesthesiol. 2005;19(3):523–38.
Meiser A, Bellgardt M, Belda J, Rohm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anaesthetic conserving device—a bench study with isoflurane and sevoflurane. J Clin Monit Comput. 2009;23(1):11–9. https://doi.org/10.1007/s10877-008-9158-4.
Bomberg H, Veddeler M, Volk T, Groesdonk HV, Meiser A (2018) Volumetric and reflective device dead space of anaesthetic reflectors under different conditions. J Clin Monit Comput (in press).
Sturesson LW, Bodelsson M, Johansson A, Jonson B, Malmkvist G. Apparent dead space with the anesthetic conserving device, AnaConDa(R): a clinical and laboratory investigation. Anesth Analg. 2013;117(6):1319–24. https://doi.org/10.1213/ANE.0b013e3182a7778e.
Sackey PV, Martling CR, Radell PJ. Three cases of PICU sedation with isoflurane delivered by the ‘AnaConDa’. Paediatr Anaesth. 2005;15(10):879–85.
Jabaudon M, Boucher P, Imhoff E, Chabanne R, Faure JS, Roszyk L, Thibault S, Blondonnet R, Clairefond G, Guerin R, Perbet S, Cayot S, Godet T, Pereira B, Sapin V, Bazin JE, Futier E, Constantin JM. Sevoflurane for sedation in acute respiratory distress syndrome. A randomized controlled pilot study. Am J Respir Crit Care Med. 2017;195(6):792–800. https://doi.org/10.1164/rccm.201604-0686OC.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. https://doi.org/10.1164/rccm.2107138.
Mapleson WW. Effect of age on MAC in humans: a meta-analysis. Br J Anaesth. 1996;76(2):179–85.
Sturesson LW, Malmkvist G, Bodelsson M, Niklason L, Jonson B. Carbon dioxide rebreathing with the anaesthetic conserving device, AnaConDa(R). Br J Anaesth. 2012;109(2):279–83. https://doi.org/10.1093/bja/aes102.
Sturesson LW, Bodelsson M, Jonson B, Malmkvist G. Anaesthetic conserving device AnaConDa: dead space effect and significance for lung protective ventilation. Br J Anaesth. 2014;113(3):508–14. https://doi.org/10.1093/bja/aeu102.
Chabanne R, Perbet S, Futier E, Ben Said NA, Jaber S, Bazin JE, Pereira B, Constantin JM. Impact of the anesthetic conserving device on respiratory parameters and work of breathing in critically ill patients under light sedation with sevoflurane. Anesthesiology. 2014;121(4):808–16. https://doi.org/10.1097/aln.0000000000000394.
Bomberg H, Meiser F, Daume P, Volk T, Sessler DI, Groesdonk HV, Meiser A. Halving the volume of AnaConDa: evaluation of a new small-volume anesthetic reflector in a test lung model. Anesth Analg. 2018 (accepted for publication).
Meiser A, Groesdonk HV, Bonnekessel S, Volk T, Bomberg H. Inhalation sedation in subjects with ARDS undergoing continuous lateral rotational therapy. Respir Care. 2017. https://doi.org/10.4187/respcare.05751.
Meiser A, Bomberg H, Lepper PM, Trudzinski FC, Volk T, Groesdonk HV. Inhaled sedation in patients with acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation. Anesth Analg. 2017. https://doi.org/10.1213/ane.0000000000001915.
Funding
The AnaConDa-50 ml reflectors were kindly provided by the manufacturer Sedana Medical, Uppsala, Sweden. The sponsor was neither involved in data acquisition, analysis, or the design of the protocol, nor did they review the submitted manuscript which was entirely prepared by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they did not receive any funding for realisation of this study. AM and TV received honoraria and travel expenses for lectures from Sedana Medical (Uppsala, Sweden). AM has been a consultant for Sedana Medical (Uppsala, Sweden). AM has received honoraria and travel expenses for lectures as well as research funding from Pall Medical (Dreieich, Germany).
Ethical approval
Informed consent and Human participants were involved.
Additional information
This manuscript contains data that will become part of the doctoral thesis of Sarah Zimmer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bomberg, H., Meiser, F., Zimmer, S. et al. Halving the volume of AnaConDa: initial clinical experience with a new small-volume anaesthetic reflector in critically ill patients—a quality improvement project. J Clin Monit Comput 32, 639–646 (2018). https://doi.org/10.1007/s10877-018-0146-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0146-z