Abstract
Purpose
To evaluate efficacy and adverse events related to inhaled sevoflurane for long-term sedation compared with standard intravenous (IV) sedation with propofol or midazolam.
Methods
Randomized controlled trial. Sixty intensive care unit (ICU) patients expected to require more than 24 h sedation were randomly assigned to one of three groups: group S, inhaled sevoflurane; group P, IV propofol; group M, IV midazolam. All patients also received IV remifentanil for goal-directed sedation (Ramsay scale and pain score) until extubation or for a maximum of 96 h. Primary end points were wake-up times and extubation delay from termination of sedative administration. Proportion of time within Ramsay score 3–4, IV morphine consumption at 24 h post extubation, hallucination episodes after end of sedation, adverse events, inorganic fluoride plasma levels, and ambient sevoflurane concentrations were recorded.
Results
Forty-seven patients were analyzed. Wake-up time and extubation delay were significantly (P < 0.01) shorter in group S (18.6 ± 11.8 and 33.6 ± 13.1 min) than in group P (91.3 ± 35.2 and 326.11 ± 360.2 min) or M (260.2 ± 150.2 and 599.6 ± 586.6 min). Proportion of time within desired interval of sedation score was comparable between groups. Morphine consumption during the 24 h following extubation was lower in group S than in groups P and M. Four hallucination episodes were reported in group P, five in group M, and none in group S (P = 0.04). No hepatic or renal adverse events were reported. Mean plasma fluoride value was 82 μmol l−1 (range 12–220 μmol l−1), and mean ambient sevoflurane concentration was 0.3 ± 0.1 ppm.
Conclusions
Long-term inhaled sevoflurane sedation seems to be a safe and effective alternative to IV propofol or midazolam. It decreases wake-up and extubation times, and post extubation morphine consumption, and increases awakening quality.
Similar content being viewed by others
Introduction
In some cases, sedation is necessary to achieve adequate comfort and security to patients in the intensive care unit (ICU) by decreasing anxiety, agitation, and pain [1]. Sedation also facilitates mechanical ventilation, and diagnostic and therapeutic procedures [1, 2]. The percentage of patients mechanically ventilated and receiving intravenous sedation has significantly increased over time [3]. No sedative agent currently available presents the features of an optimal drug: no clinically significant accumulation, no organ-dependent elimination, no prolonged wake-up time, and no hemodynamic consequences. Midazolam and propofol are the most commonly used sedative agents. Prolonged wake-up time and length of hospital stay can result from long-term infusion of these drugs. Halogenated anesthetics are used daily in operating rooms for maintenance of general anesthesia. Sedation with halogenated gases can be limited by atmospheric pollution and high costs due to the lack of rebreathing systems in the ICU. Isoflurane was studied for sedation of ventilated patients in the ICU and compared with midazolam [4, 5] or propofol [6]. Sevoflurane has a short duration of action, a brief elimination delay, and no serious side-effects, excluding the very rare malignant hyperthermia. The anesthetic conserving device (ACD) is a gas reflector that allows to limit the waste of halogenated gases in open circuits when connected between a T-tube and the patient [7]. Recently, prolonged isoflurane sedation with the ACD was evaluated for sedation in a small group of ICU patients versus midazolam [8]. Isoflurane sedation decreased wake-up times after termination of administration compared with midazolam. Sevoflurane via ACD has also been shown to be an effective and safe alternative to intravenous propofol for short-term sedation following cardiothoracic surgery [9].
To our knowledge, a goal-directed long-term sedation study comparing the three sedative drugs (i.e., inhaled sevoflurane, intravenous propofol, and intravenous midazolam) has not been done before. In the present randomized study, we tested the hypothesis that sevoflurane sedation allows a reduction in wake-up and extubation times compared with IV propofol and midazolam after long-term sedation in ventilated ICU patients. The proportion of time within Ramsay score 3–4, hallucination episodes after end of sedation, IV morphine consumption at 24 h post extubation, renal and hepatic consequences, and plasma fluoride concentration were considered secondary end points.
Materials and methods
Patients
After approval from the ethical committee, ICU patients scheduled to undergo more than 24 h of sedation for mechanical ventilation were included in this 1-year randomized study. Inclusion criteria were: age 18–80 years, weight 50–120 kg, and more that 24 h of sedation for mechanical ventilation. Exclusion criteria were: sedation started more than 6 h before inclusion; patient with septic, hemorrhagic or cardiogenic shock; head trauma patients; Glasgow scale score less than nine; pregnancy; breast-feeding; acute bleeding; pre-existing neurological disease with consciousness disorder; personal family history of malignant hyperthermia; chronic renal failure; cirrhosis with child classification C stage; severe cardiac impairment or cardiac rhythm disorders; and lack of consent (see ESM).
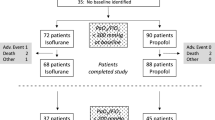
The patients were randomized into three groups: group S, sedation with inhaled sevoflurane and IV remifentanil; group P, sedation with IV propofol and remifentanil; group M, sedation with IV midazolam and remifentanil (Fig. 1). Randomization was performed by using sealed letters.
Sedation protocol
Sevoflurane was administered via the ACD (Anaconda®; Sedana Medical AB, Uppsala, Sweden). The ACD is a modified heat and moisture exchanger, placed between the Y-piece and endotracheal tube, that allows delivery of volatile anesthetics into the breathing circuit of the ICU ventilator [9]. The liquid form of sevoflurane was infused via a standard ICU syringe pump (Pilot C®; Fresenius Vial, Brezins, France) to the porous rod on the patient side of the filter. An initial bolus of 1 ml was given, and an infusion rate of 2–6 ml h−1 was initially chosen according to the patient’s minute ventilation and the manufacturer’s normogram, and then adjusted to reach a measured end-tidal concentration of sevoflurane (ETS) of 0.5% (Capnomac Ultima; Datex-Ohmeda, Helsingfors, Finland). Dose adjustments were possible within steps of 0.1% of ETS according to the Ramsay sedation scale (see Appendix in the ESM). Sedation was evaluated every 10 min by the nurse in charge of the patient until Ramsay score 3–4 was reached, then every hour. The ACD was replaced at least every 24 h. In the propofol group, IV propofol 2% was initiated at 2 mg kg−1 h−1. In the midazolam group, IV midazolam was initiated at 0.1 mg kg−1 h−1. Further adjustments were made by the patient’s nurse for Ramsay scale values of 3–4 with an assessment of the score every 10 min and adaptation in steps of 0.1 mg kg−1 h−1 for propofol and 0.05 mg kg−1 h−1 for midazolam if necessary.
Analgesia was achieved in the three groups by continuous infusion of remifentanil, started at 0.15 μg kg−1 min−1 intravenously [10, 11]. Further adjustments of remifentanil infusion were made for pain scale values of 1 (see Appendix in the ESM). Analgesia levels were evaluated every hour. Adjustments of sedatives and remifentanil are summarized in Fig. 2. The infusion rates of sevoflurane, propofol, and midazolam were recorded, as well as the number and amount of changes in doses of sevoflurane, propofol, and midazolam. The infusion rate and doses of remifentanil were recorded. Doses of remifentanil, propofol, and midazolam were reduced by 30% for patients more than 65 years old. No neuromuscular blocking agent was used. We used closed systems for tracheal aspiration in all patients.
Data collection
Analgesia and Ramsay sedation scores were evaluated every hour. Awakening quality was assessed by an awakening quality score (see Appendix in the ESM). As part of a multimodal evaluation of the patient and so that no one parameter affects another, the authors deliberately separated sedation, pain, and the quality of awakening in their evaluation by using three different scores. The analysis of the two parameters of sedation and pain was made concomitantly before changing the regimen of drugs infused (Fig. 2).
Sedation was stopped following our ICU guidelines when sedation withdrawal criteria were obtained (see ESM). Duration from termination of sedative administration until wake-up and extubation were measured. If patients were not extubated after 96 h, sedation was continued with IV drugs (propofol or midazolam associated with sufentanil or remifentanil according to physician discretion). After termination of sedation and extubation, analgesia was given to the patient using a local standardized analgesia protocol: intravenous paracetamol (1 g every 6 h), nefopam (40 mg for 30 min every 8 h), and patient-controlled analgesia with intravenous morphine to obtain a pain score less than 3 on a visual analogue scale (VAS) ranging from 0 (no pain) to 10 (high level of pain). Morphine consumption during the first 24 h following extubation was measured, and the presence of nausea and hallucinations was noted. Plasma alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT), urea, and plasma creatinine levels were measured on the first, second, third, and fifth day (see ESM). Plasma inorganic fluoride levels were measured every morning for the first 4 days in group S patients still on sevoflurane sedation. The ICU ventilators (Evita XL; Drägerwerk AG, Lübeck, Germany) in the single-bed rooms were connected to the hospital waste gas system wall outlet. Atmospheric contamination was measured in rooms of patients exposed to sevoflurane using passive lapel dosimeter sampling placed at 1 m from the ventilator. These dosimeters were later analyzed with gas chromatography. Acute Physiology and Chronic Health Evaluation (APACHE) II and Simplified Acute Physiology Score (SAPS) II scores were calculated. Continuous measurement of expiratory and inspiratory concentrations of sevoflurane was performed in group S patients. Daily total consumption of sedative agents and remifentanil were calculated. We also noted the mean Ramsay score, the proportion of time per day with Ramsay score 3–4, the mean pain score, and the number of modifications of sedative agents and remifentanil administration (after reaching the desired sedative level). Moreover, side-effects linked to ineffective sedation were noted (auto-extubation, catheter withdrawal, etc.). Duration of invasive mechanical ventilation and ICU stay were also recorded.
Statistic analysis
First we performed a descriptive analysis, with a calculation of frequencies for qualitative data and mean (±standard deviation) or median (25th–75th percentiles) for quantitative data. Then we performed a χ 2 test or a Fisher test on qualitative data when the size was too small. For qualitative data, mean comparison was calculated with analysis of variance (ANOVA) or a nonparametric Kruskal–Wallis test in case of too small size or non-Gaussian distribution of the data. The significance threshold was 0.05 for comparison of the three groups. When a significant difference appeared, a 2 by 2 comparison was performed with Bonferroni correction at the significance threshold.
Results
Sixty patients were included in the study (Fig. 1). One patient from group S was excluded because the duration of sedation was less than 24 h. Six patients were excluded from group P: one for duration of sedation less than 24 h, one for administration of ketamine, one for an adverse event from remifentanil (severe bradycardia) [12], and three for loss of follow-up data. Six patients were excluded from group M: three for administration of ketamine, and three for loss of follow-up data. Forty-seven patients were included in the final analysis. Causes of admission were surgical and nonsurgical. Demographic characteristics of the patients are summarized in Table 1. No significant difference between groups was observed for age, body mass index (BMI), sex ratio, APACHE II and SAPS II scores, duration of sedation, mechanical ventilation during the study period, and length of stay in ICU. Sedation was maintained beyond 72 h for one patient in group S, two in group P, and one in group M. All patients whose sedation was stopped before 96 h were extubated.
Sedation quality
Wake-up time was significantly shorter in group S (18.6 ± 11.8 min, P < 0.001) compared with groups P (91.3 ± 35.2 min) and M (260.2 ± 150.5 min). Wake-up time was significantly shorter in group P than in group M. Extubation delay was significantly lower in group S (33.6 ± 13.1 min, P < 0.001) compared with groups P (326.11 ± 360.2 min) and M (599.62 ± 586.95 min) (Fig. 3a, b). The number of daily modifications of sedative drugs and remifentanil administration was significantly lower in group S compared with groups P and M (Table 2). Time per day with Ramsay score 3–4 was comparable in all groups (Table 2, Fig. 3c). Time per day with mean arterial pressure (MAP) between 65 and 90 mmHg was significantly lower in groups P and M compared with group S (Table 2). Total remifentanil consumption was comparable in the three groups (Table 2). Awakening quality was significantly better in group S than in groups P and M (Table 2). In groups P and M, patients were more restless and aggressive. More patients reported hallucinations in groups P (n = 4) and M (n = 5) than in group S (n = 0, P = 0.04). The last pain score measured 24 h after termination of sedation was significantly lower in group S than in the other two groups; postoperative intravenous morphine consumption in the 24 h following extubation was also lower in group S (Table 2, Fig. 3d). No significant difference was observed between groups for PaO2/FiO2 ratios during the mechanical ventilation period (Table 2).
a Box plots of wake-up time for each group. b Box plots of time to extubation from termination for each group. c Box plots of time per day with Ramsay score 3–4 for each group. d Box plots of morphine consumption during the 24 h following extubation for each group. Sevoflurane (S), propofol (P), midazolam (M); median, 25th and 75th percentiles, 10th and 90th percentiles. Open circles represent values outside percentiles. *P < 0.05 between group S and the other two groups; §P < 0.05 between group P and group M
Safety and toxicity
No specific adverse events due to sedative drugs were noted. No patient was re-intubated in group S, versus one in group P and three in group M. None of the reintubations were related to residual sedation. The mean end-expiratory concentration of sevoflurane in the overall study period was 0.64 ± 0.13%, and the mean plasma fluoride level was 82 μmol l−1 (range 12–220 μmol l−1).
The distribution of the daily plasma fluoride value is shown in ESM. The mean ambient concentration of sevoflurane was measured at 0.3 ± 0.1 ppm at 24 h. No significant changes from baseline value for ALAT, ASAT, creatinine, and urea were detected during the course of the study, apart from a lower mean urea concentration in group S on days 3 and 4 compared with groups P and M (see ESM).
Discussion
This randomized comparative study shows that use of sevoflurane via the ACD for long-term sedation in the ICU versus intravenous propofol or midazolam significantly reduces wake-up and extubation times. The number of daily modifications in administration of hypnotic agent and remifentanil were lower with sevoflurane. Moreover, morphine consumption was reduced and awakening quality was improved in patients sedated with sevoflurane. No severe adverse event imputable to sedation was reported in any group. Inhalation of sevoflurane via the ACD seems to be a simple, sure, and safe method for long-term sedation in the ICU.
Awakening and extubation delays
Drug accumulation and oversedation is one of the main concerns with intravenous sedation in the ICU. Kress et al. [13] reported that prolonged wake-up times lead to increased length of stay in the ICU. Different strategies have been suggested to avoid accumulation of sedative drugs, such as sequential administration of midazolam and propofol before weaning [14], daily interruption of sedation [13, 15] or use of sedation protocols implemented by nurses [16]. According to our results, sevoflurane could be one solution to limit oversedation and prolongation of ICU stay.
We reported a mean extubation delay shorter than that described in previous studies for propofol and midazolam sedation. In one study, mean values of 35 h for propofol and 98 h for midazolam were reported after 141 h of sedation in 108 ICU patients [17]. In another study, weaning time was 24 h with propofol and 81 h with midazolam after 115 h of sedation [18]. These differences could be explained by the properties of remifentanil. The onset time of remifentanil is very short, which allows quick titration; fentanyl or sufentanil were used in previous studies. Moreover, we used a goal-directed protocol with a desired interval of depth of sedation lower than in these studies. We also followed accurate weaning and extubation protocols, i.e., a spontaneous breathing trial test with a pressure support ventilation period of 20 min, and 10 min of T-tube spontaneous breathing before extubation.
In a similar long-duration goal-directed sedation study (52 h) (12–96) comparing inhaled isoflurane via the ACD versus intravenous midazolam, Sackey et al. [8] reported a time to extubation with isoflurane of 10 ± 5 min. Our results are quite similar to those of Rohm et al. [9] after 8 h of sedation with sevoflurane or propofol in surgical patients. The median time to extubation was 21.5 (8–46) min for sevoflurane and 150.5 (69–299) min in the propofol group, and length of hospital stay was also shorter [9]. The decrease in wake-up time obtained in our sevoflurane group allows better planned extubation by the ICU physician. In the other groups, the ICU physician needs to be prepared for extubation for much longer periods of time. Furthermore, the unnecessarily long wake-up times lead to increased diagnostic testing to rule out intracranial events in some ICU patients [15]. However, in our study, the decrease in wake-up and extubation times in the sevoflurane group did not lead to a decrease in overall mechanical ventilation and duration of ICU stay, as previously reported in studies after long-term sedation [19]. The quality of awakening was much better in the patients in group S. We reported better awakening scores and fewer postsedation hallucination episodes in patients in group S. Our results are in accordance with those reported by Sackey et al. [20], who showed that isoflurane sedation in ICU patients resulted in fewer delusional memories or hallucinations from the ICU. Those symptoms are associated with post-ICU depression or posttraumatic stress disorder [8, 21].
Antihyperalgesic effect of sevoflurane
We found a better awareness quality, noted as better awakening quality scores and less hallucination occurrence, in the sevoflurane group. Furthermore, the fact that the last recorded pain score and the postextubation intravenous morphine consumption were significantly lower in group S (although remifentanil consumption was similar to groups P and M) could be explained by the hypothesis that sevoflurane could have N-methyl-d-aspartate (NMDA) antagonist activity. Several cellular studies show an antagonist action of volatile anesthetics and opioids on NMDA receptors [22, 23]. Volatile anesthetics could generate noncompetitive inhibition of these receptors, whereas opioids would activate them in a dose-dependent way. Low isoflurane concentrations (<1.0%) reversed fentanyl-induced hyperalgesia in rats [24]. Moreover, volatile anaesthetics (isoflurane, sevoflurane, and desflurane) inhibit functioning of NMDA receptors expressed recombinantly in Xenopus oocytes, in a reversible, concentration-independent, voltage-insensitive manner [22]. Sackey et al. [8] also found that mean morphine consumption tended to be higher in the midazolam group than in the isoflurane group. Our study seems to be the first to report the observation of an antihyperalgesic effect of sevoflurane during long-term sedation in ICU patients.
Safety and toxicity
Methoxyflurane was withdrawn from clinical use after reversible subclinical renal dysfunction was observed when serum organic fluoride levels due to methoxyflurane metabolism rose above 50 μM. Clinically observed dysfunctions occurred at levels >100 μM [25]. In our study, no element of toxicity induced by long-term sedation with sevoflurane was found; in particular, no renal toxicity due to sevoflurane was observed, whereas plasma fluoride levels often exceeded the classically accepted threshold of 50 μM.
Limits
Some limitations of our study deserve comment. First, a double-blind design was not applicable to our study, so we cannot ignore that bias could have affected the subjective assessment of the nursing staff. Indeed, although the study used a randomized design, we cannot exclude a “Hawthorne effect” (protocol influence due to a new “experimental procedure which can not be blinded”) in the experimental group (i.e., sevoflurane group). The device of sevoflurane administration was new, and may increase the physicians’ and nurses’ responsibilities for control of sedation and collected data. However, nearly all the data were collected by our ICU nurses, none of whom expressed a preference for either sevoflurane, propofol or midazolam use, during their care of patients in this study. Second, we did not use a bispectral index (BIS) to optimize the level of sedation. Two reasons explain this: we did not use myorelaxants in our patients [26], and BIS monitoring for sedation is not recommended for ICU patients [27]. Third, similarly to sedation accurately evaluated by the clinical Ramsay score, which is a heteroevaluation, we cannot use an objective tool for pain evaluation. In the present study, we used a local pain scale which demonstrated a moderate to good interrater reliability with the validated behavioral pain scale [2, 28] (data not shown). Finally, the studied population is young and mainly post trauma, so our results should be applied with caution to a larger population.
Our results suggested sevoflurane sedation using the ACD to be an effective and safe alternative to the usual intravenous propofol- or midazolam-based regimen. Sevoflurane provided sedation quality comparable to propofol and midazolam, but with decreased wake-up times and extubation delay.
References
Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, Binhas M, Genty C, Rolland C, Bosson JL (2007) Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 106:687–695; quiz 891-682
Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J (2009) Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post Hoc analysis of the DOLOREA study. Anesthesiology 111:1308–1316
Wunsch H, Kahn JM, Kramer AA, Rubenfeld GD (2009) Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med 37:3031–3039
Kong KL, Willatts SM, Prys-Roberts C (1989) Isoflurane compared with midazolam for sedation in the intensive care unit. BMJ 298:1277–1280
Spencer EM, Willatts SM (1992) Isoflurane for prolonged sedation in the intensive care unit; efficacy and safety. Intensive Care Med 18:415–421
Millane TA, Bennett ED, Grounds RM (1992) Isoflurane and propofol for long-term sedation in the intensive care unit. A crossover study. Anaesthesia 47:768–774
Enlund M, Lambert H, Wiklund L (2002) The sevoflurane saving capacity of a new anaesthetic agent conserving device compared with a low flow circle system. Acta Anaesthesiol Scand 46:506–511
Sackey PV, Martling CR, Granath F, Radell PJ (2004) Prolonged isoflurane sedation of intensive care unit patients with the anesthetic conserving device. Crit Care Med 32:2241–2246
Rohm KD, Wolf MW, Schollhorn T, Schellhaass A, Boldt J, Piper SN (2008) Short-term sevoflurane sedation using the anaesthetic conserving device after cardiothoracic surgery. Intensive Care Med 34:1683–1689
Rozendaal FW, Spronk PE, Snellen FF, Schoen A, van Zanten AR, Foudraine NA, Mulder PG, Bakker J (2009) Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: a centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med 35:291–298
Spies C, Macguill M, Heymann A, Ganea C, Krahne D, Assman A, Kosiek HR, Scholtz K, Wernecke KD, Martin J (2010) A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med
Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC (2007) Remifentanil for general anaesthesia: a systematic review. Anaesthesia 62:1266–1280
Kress JP, Vinayak AG, Levitt J, Schweickert WD, Gehlbach BK, Zimmerman F, Pohlman AS, Hall JB (2007) Daily sedative interruption in mechanically ventilated patients at risk for coronary artery disease. Crit Care Med 35:365–371
Saito M, Terao Y, Fukusaki M, Makita T, Shibata O, Sumikawa K (2003) Sequential use of midazolam and propofol for long-term sedation in postoperative mechanically ventilated patients. Anesth Analg 96:834–838, table of contents
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342:1471–1477
Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH (1999) Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 27:2609–2615
Barrientos-Vega R, Mar Sanchez-Soria M, Morales-Garcia C, Robas-Gomez A, Cuena-Boy R, Ayensa-Rincon A (1997) Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med 25:33–40
Carrasco G, Molina R, Costa J, Soler JM, Cabre L (1993) Propofol vs midazolam in short-, medium-, and long-term sedation of critically ill patients. A cost-benefit analysis. Chest 103:557–564
Walder B, Elia N, Henzi I, Romand JR, Tramer MR (2001) A lack of evidence of superiority of propofol versus midazolam for sedation in mechanically ventilated critically ill patients: a qualitative and quantitative systematic review. Anesth Analg 92:975–983
Sackey PV, Martling CR, Carlsward C, Sundin O, Radell PJ (2008) Short- and long-term follow-up of intensive care unit patients after sedation with isoflurane and midazolam–a pilot study. Crit Care Med 36:801–806
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291:1753–1762
Hollmann MW, Liu HT, Hoenemann CW, Liu WH, Durieux ME (2001) Modulation of NMDA receptor function by ketamine and magnesium. Part II: interactions with volatile anesthetics. Anesth Analg 92:1182–1191
Matute E, Lopez-Garcia JA (2003) Characterisation of sevoflurane effects on spinal somato-motor nociceptive and non-nociceptive transmission in neonatal rat spinal cord: an electrophysiological study in vitro. Neuropharmacology 44:811–816
Richebe P, Rivalan B, Rivat C, Laulin JP, Janvier G, Maurette P, Simonnet G (2009) Effects of sevoflurane on carrageenan- and fentanyl-induced pain hypersensitivity in Sprague-Dawley rats. Can J Anaesth 56:126–135
Cousins MJ, Mazze RI, Kosek JC, Hitt BA, Love FV (1974) The etiology of methoxyflurane nephrotoxicity. J Pharmacol Exp Ther 190:530–541
Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B (2003) Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology 99:9–17
Sackey PV (2008) Frontal EEG for intensive care unit sedation: treating numbers or patients? Crit Care 12:186
Chanques G, Sebbane M, Barbotte E, Viel E, Eledjam JJ, Jaber S (2007) A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology 107:858–860
Acknowledgments
Support was provided only from institutional sources.
Conflict of interest
No conflict of interest was declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Mesnil and X. Capdevila contributed equally to this study.
This article is discussed in the editorial available at doi:10.1007/s00134-011-2214-4.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mesnil, M., Capdevila, X., Bringuier, S. et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med 37, 933–941 (2011). https://doi.org/10.1007/s00134-011-2187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2187-3