Abstract
New rare earth (RE) carbodiimides having the formula RE8O(CN2)10Br2 were synthesized by solid-state metathesis reactions. Structure determinations and refinements, based on single-crystal X-ray diffraction data, are performed with the space group P͞3c1. Homologous compounds with RE = La, Ce, Pr, and Nd are assigned isotypically. The refined crystal structure appears most unusual for rare earth carbodiimide compounds. The structure is characterized by the formally neutral [RE6O(NCN)8] fragment that is tightly interconnected by carbodiimide ions into a network structure, and contains additional RE3+ ions in the structure. Crystals of the compounds show characteristic colors of their corresponding RE3+ ions and behave stable in air. The presence of (CN2)2− ions was confirmed by infrared spectroscopy. Ce8O(CN2)10Br2 is a photoluminescent material that is showing a broad and efficient emission band in the yellow region of the visible spectrum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A tremendous number of rare earth (RE) dinitridocarbonates, usually subdivided into carbodiimides (N = C = N)2− and cyanamides (N≡C-N)2− have been reported. The vast growth of this field originates from the development of a most useful synthesis route for this type of compounds, namely the solid-state metathesis reaction [1].
Solid-state metathesis (SSM) reactions have shown some major advantages compared to other (often explorative) solid-state reactions. For example, a) they can be performed at moderate heating temperatures to stabilize thermally metastable compounds, b) they proceed quickly, so that the course of a reaction or a sequence of (new) products can be closely monitored by differential thermal analysis (DTA) or differential scanning calorimetry (DSC) [2, 3]. A systematic study of rare earth carbodiimides has been performed for a whole series of RE2(CN2)3 [4,5,6] compounds following exothermic SSM reactions, in which a rare earth halide is reacted with lithium carbodiimide:
This basic reaction (1) was then modified by different proportions of additional reaction partners to yield RECl(CN2) [7] and LiRE(CN2)2 [8, 9] compounds. Subsequently, diverse anions such as oxide, nitride, silicate, and others were included into SSM reactions [10, 11] to produce several mixed anion compounds including the metal oxide carbodiimides RE2O2(CN2) [12,13,14] and RE2O(CN2)2 [15].
A novelty was the development of compounds containing the complex tetracyanamidosilicate anion in ARE[Si(CN2)4] (A = K, Rb, Cs, and RE = La, Ce, Pr, Nd, Sm, Eu, Gd) as a structural derivative of the (SiO4)4− ion [16]. Lanthanide (Ln) doped compounds of RE2(CN2)3:Ln [17], RE2O2(CN2):Ln [18] and ARE[Si(CN2)4]:Ln have been reported for their photoluminescent properties [17, 19]. Moreover, tetracyanamidogermanate compounds crystallizing with a non-centrosymmetric space group have shown luminescent as well SHG properties [20, 21].
Rare earth carbodiimide compounds typically appear with layered arrangements of their constituents, in which metal atoms are situated in every other layer. Carbodiimide ions in interlayers appear as (N = C = N)2– ions, with bond lengths near 120 pm and N–C-N bond angles near 180°, as observed in the structure of lithium carbodiimide [22].
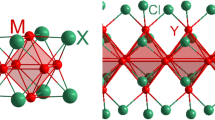
Rare earth carbodiimide nitrides RE2Cl(CN2)N with RE = La, Ce, [23] and RE2I(CN2)N [24] with RE = La, Gd were reported to show a new structural pattern, based on linear chains of edge-bridging [RE6] octahedra with the [RE2RE4/2(NCN)4/4N4/2] fragment (Fig. 1). This pattern appears closely related to the arrangement of atoms in the structure of the metal-rich compound Gd2Cl3 [25] and parallels the pattern of well-known octahedral transition metal clusters of the [M6X8] type, with M = metal, and X being chalcogenide or halide.
In this contribution we describe new compounds with a structure based on oxygen-centred [RE6O] octahedra. The synthesis and crystal structure of RE8O(CN2)10Br2 compounds is reported for the isotypic series RE = La, Ce, Pr and Nd. Infrared and Photoluminescence studies are reported for Ce8O(CN2)10Br2.
Experimental Materials and Methods
Synthesis
All manipulations of the starting materials were performed in a glovebox under dry argon atmosphere.
Preparation of Li2(CN2) and REOBr
Li2(CN2) used in reactions was obtained by reacting Li3N (Alfa Aesar 99.4%) and C3N3(NH2)3 in 2:1 molar ratio. The mixture was heated at 270 °C within an open corundum boat under flowing argon and kept at this temperature for 20 min. Afterwards, the temperature was increased to 330 °C with a rate of 0.5 °C/min and kept for 10 min. The mixture was then heated up to 600 °C with the rate of 3.5 °C/min, kept at this temperature for 30 min, and then cooled to room temperature with the same rate. After the reaction, the produced material was transferred into a glove box (Ar). The purity of the material was checked by powder X-ray diffraction.
REOBr prepared from RE2O3 (RE = Nd (99.99%), La (99.99%), Ce (99.95%) and Pr (99.95%) (Spécialités Chimiques, phosphor grade) and ammonium bromide (Alfa-Aesar, 425 °C subl.) in 1: 2.5 molar ratio. The mixture was heated with a rate of 10 °C/min to 900 °C in an open corundum boat under argon flow, kept at this temperature for 2 h, and cooled to room temperature with a rate of 10 °C/min. The purity of the material was inspected by powder X-ray diffraction.
Synthesis of RE2O(CN2)10Br2 (RE = La, Ce, Pr, Nd)
All compounds were prepared following the same procedure: A mixture of REBr3 (Sigma-Aldrich 99.99%), REOBr and Li2(CN2) was ground in an agate mortar following a molar ratio of 7:1:10 (total mass ca. 200 mg). The mixture was loaded and fused into evacuated silica ampoule and heated to 500 °C with a heating rate of 2 °C/min, kept at this temperature for 48 h, and then cooled to room temperature with a rate of 0.1 °C/min. The product was washed, first with ethanol and then dried with acetone. RE2O(CN2)10Br2 compounds were obtained in quantitative yields as air-stable materials.
Single-Crystal X-Ray Diffraction
Single crystals of RE8O(CN2)10Br2 (RE = La, Pr, Nd) were mounted on a micro loop and placed onto a Rigaku XtaLAB Synergy-S single-crystal X-ray diffractometer equipped with a HyPix-6000HE detector and monochromated Mo-Kα radiation (λ = 71.073 pm). The X-ray intensities were corrected for absorption with numerical method. The structure was solved by direct methods with OLEX software [26]. A cooling was applied with a stream of nitrogen to T = 100(1) K using a cryostream cooler (series 800, Oxford Cryosystems) and maintained at this temperature during the measurement.
Powder X-Ray Diffraction
Reaction products were inspected by powder X-ray diffraction (StadiP, Stoe, Darmstadt, Ge-monochromated Cu-Kα1 radiation) in the range 7 < Θ < 60°. The structure of Ce8O(CN2)10Br2 was refined using the Fullprof-Suite [27].
Infrared Spectroscopy
The samples were prepared in a KBr pellet and the infrared vibrational spectrum of Ce8O(CN2)10Br2 was recorded with a Bruker Vertex 70 spectrometer within the range of 400—4000 cm−1.
Energy-Dispersive X-ray (EDX) Measurement
The EDX spectroscopy data were collected with a Hitachi SU8030 scanning electron microscope and a Bruker QUANTAX 6G EDX detector. Crystals of Ce8O(CN2)10Br2 were fixed on a carbon tape.
Luminescence Spectroscopy
Excitation and emission spectra of Ce8O(CN2)10Br2 were recorded with a fluorescence spectrometer FLS920 (Edinburgh Instruments) equipped with a 450 W ozone-free xenon arc lamp (OSRAM) and a sample chamber installed with a mirror optic for powder samples. For detection, a R2658P single-photon counting photomultiplier tube (Hamamatsu) was used. All luminescence spectra were recorded with a spectral resolution of 1 nm, a dwell time of 0.4 s in 1 nm steps, and three repeats.
Results and Discussion
Synthesis of RE8O(CN2)10Br2
RE8O(CN2)10Br2 compounds were synthesized by solid-state metathesis reaction from appropriate mixtures of REBr3, Li2(CN2), and REOBr following reaction (2). A typical mixture was heated with a rate of 2 °C/min to 500 °C, kept at this temperature for one week and then cooled with a rate of 0.1 °C/min.
The products of reactions according to (2) appeared as crystalline powders of new RE8O(CN2)10Br2 compounds with the metathesis salt LiBr, as detected by powder XRD diffraction. LiBr was washed out with ethanol and the product was dried with acetone. Crystal powders of RE8O(CN2)10Br2 appear transparent with the characteristic body color of the respective RE3+ ion (Fig. 2).
Crystal Structure of Ce8O(CN2)10Br2
The crystal structure of Ce8O(CN2)10Br, as described exemplarily herein for the series of isotypic RE8O(CN2)10Br2 compounds (RE = La, Ce, Pr, Nd) were refined from single-crystal X-ray for RE = La, Pr, Nd, and for RE = Ce from powder diffraction (XRD) data with the trigonal space group P͞3c1 and lattice parameters of a = 926.481(1) and c = 1594.44(1) pm (Table 1).
The most remarkable feature in the crystal structure is the centrosymmetric [Ce6O] cluster core (Ce1) that is interconnected by carbodiimide (N = C = N) ions into a complex network structure. Two further isolated Ce3+ ions (Ce2) are present in the structure. The coordination pattern of the cluster and the connectivity pattern in the structure is described as follows:
The [Ce6O] cluster core is represented by an almost octahedral arrangement of cerium ions with Ce1-Ce1 distances of 387.5(2) and 385.7(2) pm, centered by an oxygen atom with six Ce1-O distances of 273.4(1) pm. Carbodiimide ions are capping the eight faces of the octahedral cerium cluster via terminal nitrogen atoms to form a [Ce6O(NCN)8] arrangement (Fig. 3, top).
Assembly of the ligand environment of the [RE6O] core given in three steps. Top: Ligand environment of the [RE6O] cluster displaying only face-capping, respectively inner (i) ligands of [(RE6O)(NCN)i−i2(NCN)6i−a] indicating two distinct connectivities (i−i and i−a) with adjacent clusters. Center: Environment of [Ce6O], displaying only apical or outer (a) ligands as [RE6O(NCN)a−i6/2(NCN)a−a6/2]. Bottom: Complete environment as [RE6O(CN2)i−i2/2(CN2)i−a6/2(CN2)a−i6/2(CN2)a−a6/2]. Lines between metal atoms are guidelines for the eye and do not represent bonding
The [(Ce6O)(NCN)8] unit can be compared with the well-known [M6X8] type cluster, typically made up of six metal atoms (M) and eight chalcogenide or halide atoms (X). Within the given cluster nomenclature [28] these (X) ligands are assigned as inner (i) ligands, or as i−i when there is a bridging connectivity between inner (or face-capping) positions of adjacent clusters, or as (i−a) when the connectivity of an inner ligand is directed to the outer (a) (or apical) position of adjacent clusters. Following this notation, we define a central structure element as [Ce6O(NCN)i−i2(NCN)4i−a] in which the two inner-inner (i−i) connections generate chains of clusters parallel to the threefold (c) axis in the structure (Fig. 4).
The (i−a) connectivity of the shared carbodiimide ion is converted into a (a−i) connectivity when looking from the outer (a) position of the adjacent cluster; but we must keep in mind that crystallographically there is only one independent cluster in the structure. A view of the outer (apical) positions of the cluster is displayed in the center of Fig. 3. Each cerium ion of the cluster has two carbodiimide ligands in apical positions, one (a−a) and one (a−i). All carbodiimide ligands together are shown in Fig. 3 (bottom), to resemble the full coordination environment of the cluster and its connectivity pattern via three shared carbodiimide ions as [(Ce6O)(CN2)i−i2/2(CN2)i−a6/2(CN2)a−i6/2(CN2)a−a6/2].
Ce–N distances range between 244.2(3) and 272.2(3) pm for Ce1. All C–N distances of the three crystallographically distinct carbodiimide ions range between 120.4(2) pm and 122.9(4) pm.
Linear chains of (NCN) bridged clusters, shown in Fig. 4, are running parallel to the trigonal axis (c), along the cell edges of the primitive unit cell of Ce8O(CN2)10Br2. Two more Ce3+ ions (Ce2) and two bromide ions are contained in the structure to yield Ce2[Ce6O(CN2)i−i2/2(CN2)i−a6/2(CN2)a−i6/2(CN2)a−a6/2]Br2. The two “isolated” cerium ions (Ce2) in the structure are situated in a seven-fold coordination environment displayed in Fig. 5. They are surrounded by six nitrogen atoms of carbodiimide ions (Ce2-243.8(3) and 245.2(4) pm), which connect into other positions of the cluster and one bromide ion (Ce2-Br: 307.8(4) pm). The projection in Fig. 6 is showing the complete arrangement in the unit cell.
In general, the notation of given rare earth compounds as clusters is fundamentally different from the description used for transition-metal cluster compounds. The term “cluster” was originally used for the description of compounds containing several metal atoms connected by metal-to-metal bonds [29]. Today, the term is also used for compounds in which several metal atoms are connected through bridging atoms or ligands. Sometimes the term “coordination cluster “ is used for these compounds [30]. In this respect, it must be considered that connecting lines drawn herein between metal atoms (octahedra) in figures just represent guidelines for the eye.
The composition of Ce8O(CN2)10Br2 was confirmed by EDX analyses giving a Ce: Br ratio of 8.01: 2.0.
Isotypic compounds RE8O(CN2)10Br2 with RE = La, Ce, Pr and Nd were all obtained under corresponding conditions and refined on basis of single-crystal or powder X-ray diffraction data.
Their crystal data and some selected data of the structure refinement are summarized in Table 1. The purity of Ce8O(CN2)10Br2 and Nd8O(CN2)10Br2 was confirmed by Rietveld refinement (Figure S1 and S2, Table S1).
The [(RE6O)(NCN)8] fragment with eight inner carbodiimide ions in the structure of RE8O(CN2)10Br2 compares well with that of [RE6(NCN)4N4] in RE2Cl(CN2)N compounds (Fig. 1) without an interstitial atom in the [RE6] octahedron. This is consistent with an essentially salt-like character of both type of compounds, RE8O(CN2)10Br2 and RE2Cl(CN2)N.
Other examples of an oxo-centered rare earth cluster compounds have been reported as products crystallized from aqueous solutions, such as [(Nd6O)(OH)8(H2O)24]8+, [31, 32] [Nd6(μ6-O)(μ3-OH)8] [32] or the mixed valent Ce(III)/(IV) cluster ion [CeIV38-nCeIIInO56-(n+1)(OH)n+1Cl51(H2O)11]10− (n = 1–24) [33].
Infrared Studies
The crystal structure of [(RE6O)(CN2)i−i2/2(CN2)i−a6/2(CN2)a−i6/2(CN2)a−a6/2] is characterized by three distinct carbodiimide ions. Connectivities between adjacent cluster faces are accomplished by symmetrical N2-C2-N2 bridges along the threefold axis displayed as i−i bridges in Fig. 4. These distance amount to dN-C = 122.9(2) pm in Ce8O(CN2)10Br2. Another symmetrical carbodiimide group N1-C1-N1 connects vertices of adjacent clusters at dN-C = 120.4(2) pm, assigned as a−a bridges in Fig. 3 (top). Finally, the slightly distorted group N3-C3-N4 with dN-C-N = 122.4(4) pm and 122.9(4) pm reveals an i−a connectivity indicated in top of Fig. 3. The relative proportions of these carbodiimides bridges in the structure of Ce8O(CN2)10Br2 are 3:1:6.
The infrared spectrum recorded for Ce8O(CN2)10Br2 shows typical features of the presence of carbodiimides ions. The asymmetric stretching of the [N = C = N]2− ion is split into two signals within the given resolution of our spectrometer, centered at 1978 cm−1 and 2054 cm−1. The bending modes are observed around 680 cm−1, and bands at lower wavenumbers, referring to metal-nitrogen bonding, all displayed in Fig. 7 [34, 35].
Luminescence of Ce8O(CN2)10Br2
The diffuse reflection spectrum of the yellow–brown µ-crystalline powder of Ce8O(CN2)10Br2 shows an absorption band at around 360 nm, which is attributed to an absorption process caused by an interconfigurational 4f – 5d transition of the isolated Ce3+ ions (Fig. 8). This absorption band is overlaid by further absorption features, since the reflectance steadily decreases from 360 nm down to 250 nm. The gradual decrease in reflection in the 360—800 nm range is unstructured and points to the presence of structural defects [36].
To reveal the nature of the strong yellow luminescence observed by excitation with a UV radiation source, the emission spectrum upon 355 nm excitation and the respective excitation spectrum monitored for the 545 nm photoluminescence was recorded (Fig. 9).
The emission spectrum shows a very broad emission band centered at 545 nm with a FWHM of 0.65 eV (4500 cm−1). Such a large width is typical for Ce3+ centered luminescence due to the ground state splitting of the [Xe]4f1 configuration into the states 2D7/2 and 2D5/2 by spin–orbit coupling [37]. Monitoring the photoluminescence at 545 nm reveals an excitation spectrum with a peak at 355 nm, which refers to a Stokes Shift of about 1.24 eV (10,000 cm−1). Such a large Stokes Shift can be explained due to large relaxation of the excited state. Such finding can be attributed to the “loose” coordination sphere of the isolated Ce3+ ions, since they are seven-fold coordinated by six carbodiimide ligands and a single bromide ligand with rather large bond length.
We assume that the [Ce6O(CN2)8] unit is not luminescent even though the well-known similar [M6X8] type cluster (with M = Mo, W) are strongly luminescent with decay times in the range of 2 – 500 µs [38, 39]. This is confirmed by the decay curve of the emission at 545 nm upon excitation by a pulsed 375 nm laser diode.
The obtained decay curve can be nicely fit by a biexponential function, whereby the obtained decay constants are 2.7 and 5.0 ns, which is rather short, since Ce3+ activated materials show typical decay times between 20 and 80 ns (Fig. 10). However, a possible explanation is a charge transfer quenching process, between the excited state of the isolated Ce3+ ions and the [Ce6O(CN2)8] unit. Such a process resulting in a tremendous decline of the decay time of Ce3+ has been observed for PrBr3:Ce3+ [40].
Conclusions
The vast majority of metal dinitridocarbodiimides have been prepared by straight forward SSM reactions. Their crystal structures are characterized by alternating layered arrangements of metal and dinitridocarbonate ions. Similar compounds such as dinitridocarbonate-oxides REO(CN2)2 and REO2(CN2)[15] can be prepared accordingly, with the employment different amounts of an oxide source in reactions.
The herein reported isotypic RE8O(CN2)10Br2 compounds with RE = La, Ce, Pr, Nd introduce a new structure pattern with an oxygen centered RE6O cluster core. The [RE6O(NCN)8] structure can be well compared to the previously reported condensed [RE6(NCN)4N4] cluster chain in RE2Cl(CN2)N, and finally with metal-rich compounds RE2Cl3.
Carbodiimide bridges connect RE6O clusters and isolated RE ions in RE8O(CN2)10Br2 to form a tight network structure of air-stable compounds. Crystalline powder of Ce8O(CN2)10Br2 is showing a broad yellow photoluminescence.
References
R.H.-J. Meyer (2010). Dalton Trans. 39, 5973.
T. B. Tang and M. M. Chaudhri (1980). J. Therm. Anal. Calorim. 18, 247.
A. Mos-Hummel, M. Ströbele, and H.-J. Meyer (2016). Eur. J. Inorg. Chem. 2016, 4234.
M. Neukirch, S. Tragl, and H.-J. Meyer (2006). Inorg. Chem. 45, 8188.
O. Reckeweg, T. Schleid, and F. J. DiSalvo (2007). ChemInform. 38, 658.
Y.-C. Wu, T.-M. Chen, C.-H. Chiu, and C.-N. Mo (2010). J. Electrochem. Soc. 157, 342.
R. Srinivasan, J. Glaser, S. Tragl, and H.-J. Meyer (2005). Z Anorg Allg Chem. 631, 479.
L. Unverfehrt, M. Ströbele, and J. Glaser (2009). Inorg. Chem. 635, 1947.
M. Kubus, R. Heinicke, M. Ströbele, D. Enseling, T. Jüstel, and H.-J. Meyer (2015). Mater. Res. Bull. 62, 37.
O. Reckeweg and F. J. DiSalvo (2000). Angew. Chem. Int. Ed. 39, 412.
O. Reckeweg and F. J. DiSalvo (2001). Z Anorg Allg Chem. 627, 371.
Y. Hashimoto, M. Takahashi, S. Kikkawa, and F. Kanamaru (1995). J. Solid State Chem. 114, 592.
Y. Hashimoto, M. Takahashi, S. Kikkawa, and F. Kanamaru (1996). J. Solid State Chem. 125, 37.
M. Li, W. Yuan, J. Wang, C. Gu, and H. Zhao (2007). Powder Diffr. 22, 59.
R. Srinivasan, S. Tragl, and H.-J. Meyer (2005). Z. Anorg. Allg. Chem. 631, 719.
M. Kubus, J. Glaser, A. Kłonkowski, and H.-J. Meyer (2010). Z Anorg Allg Chem. 636, 991.
J. Glaser, L. Unverfehrt, H. Bettentrup, G. Heymann, H. Huppertz, T. Jüstel, and H.-J. Meyer (2008). Inorg. Chem. 47, 10455.
L. T. Wang, S. L. Yuan, Y. X. Yang, F. Chevire, F. Tessier, and G. R. Chen (2015). Opt. Mater. Express. 5, 2616.
J. Glaser, H. Bettentrup, T. Jüstel, and H.-J. Meyer (2010). Inorg. Chem. 49, 2954.
M. Kalmutzki, D. Enseling, J. E. C. Wren, S. Kroeker, V. V. Terskikh, T. Jüstel, and H.-J. Meyer (2013). Inorg. Chem. 52, 12372.
K. Dolabdjian, C. Schedel, D. Enseling, T. Jüstel, and H.-J. Meyer (2017). Z Anorg Allg Chem. 643, 488.
M. G. Down, M. J. Haley, P. Hubberstey, R. J. Pulham, and A. E. Thunder (1978). J. Chem. Soc. Dalton Trans. 10, 1407.
R. Srinivasan, M. Ströbele, and H.-J. Meyer (2003). Inorg. Chem. 42, 3406.
D. Dutczak, A. Siai, M. Ströbele, D. Enseling, T. Jüstel, and H.-J. Meyer (2020). Eur. J. Inorg. Chem. 2020, 3954.
A. Simon, N. Holzer, and H. Mattusch (1979). Z Anorg Allg Chem. 456, 207.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann (2009). J. Appl. Crystallogr. 42, 339.
J. Rodríguez-Carvajal (1993). J. Condens. Matter Phys. 192, 55.
H. Schäfer and H. G. Schnering (1964). Angew. Chem. 76, 833.
F. A. Cotton, N. F. Curtis, C. B. Harris, B. F. G. Johnson, S. J. Lippard, J. T. Mague, W. R. Robinson, and J. S. Wood (1964). Science 145, 1305.
A. T. Wagner and P. W. Roesky (2016). Eur. J. Inorg. Chem. 2016, 782.
A.-V. Mudring and A. Babai (2005). Z Anorg Allg Chem. 631, 261.
D.-S. Zhang, B.-Q. Ma, T.-Z. Jin, S. Gao, C.-H. Yan, and T. C. W. Mak (2000). New J Chem. 24, 61.
J. N. Wacker, A. S. Ditter, S. K. Cary, A. V. Murray, J. A. Bertke, G. T. Seidler, S. A. Kozimor, and K. E. Knope (2022). Inorg. Chem. 61, 193.
G. Rapi and G. Sbrana (1971). J. Am. Chem. Soc. 93, 5213.
S. T. King and J. H. Strope (1971). J. Chem. Phys. 54, 1289.
F. Urbach (1953). Phys. Rev. 92, 1324.
D. J. Robbins (1979). J. Electrochem. Soc. 126, 1550.
M. Ströbele, T. Jüstel, H. Bettentrup, and H.-J. Meyer (2009). Z Anorg Allg Chem. 635, 822.
A. W. Maverick, J. S. Najdzionek, D. MacKenzie, D. G. Nocera, and H. B. Gray (1983). J. Am. Chem. Soc. 105, 1878.
M.D. Birowosuto, P. Dorenbos, C.W.E.v. Eijk, K.W. Krämer, H.U. Güdel (2007). J. Condens. Matter Phys. 19, 256209.
Acknowledgements
Funding by the Deutsche Forschungsgemeinschaft (DFG) through grant ME 914/25–2 is gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siai, A., Brand, CD., Ströbele, M. et al. Carbodiimide Bridged Network Structure of [RE6O(NCN)6] Clusters in the Structure of RE8O(CN2)10Br2, RE = La, Ce, Pr, Nd. J Clust Sci 34, 1001–1008 (2023). https://doi.org/10.1007/s10876-022-02286-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02286-7