Abstract
Y4O2(CN2)3Cl2 and some isotypic rare earth (RE) carbodiimides RE4O2(CN2)3Cl2 (RE = Sm, Gd, Tb) were prepared by solid-state metathesis reaction between RECl3, REOCl and Li2CN2. The crystal structure of Y4O2(CN2)3Cl2 was solved and refined from single-crystal XRD data with the monocline space group C2/m. Homologous compounds with RE = Sm, Gd, Tb were assigned isotypically by means of XRD powder diffraction. The structure of Y4O2(CN2)3Cl2 is characterized by one-dimensional cluster chains which are showing a striking resemblance to the trans-edge bridging RE6-octahedra in the structures of Y2Cl3, β-Y2Cl3N, and RE2N(CN2)Cl, despite different numbers of cluster electrons. Crystals of RE4O2(CN2)3Cl2 appear as transparent rods. The presence of (NCN)2− ions is confirmed by infrared spectroscopy. Y4O2(CN2)3Cl2, doped with Ce3+ is a photoluminescent material that is showing an emission band in the orange region (λ = 550 nm) of the visible spectrum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A broader chemistry of rare-earth (RE) dinitridocarbonates was developed after the discovery of the series of RE2(CN2)3 [1] compounds, that were synthesised by solid-state metathesis (SSM) [2] reactions. The dinitridocarbonate ion is often assigned as carbodiimide, with bond lengths within the (N=C=N)2- ion near 120 pm and N–C–N bond angles near 180°, as observed in the structure of lithium carbodiimide [3], or as cyanamide (N–C≡N)2– with distinct N–C–N bond lengths related to those in cyanamide (H2NCN) itself.
The majority of crystal structures, like RE2(CN2)3 [1, 4], RECl(CN2) [5], RE2O2(CN2) [4, 6, 7], and LiRE(CN2) [8] are forming layered arrangements of their constituents, in which metal atoms and (NCN)2− ions are situated in alternating layers. These compounds appear as transparent materials, of which Y2(CN2)3:Ce has revealed promising photoluminescence properties [9].
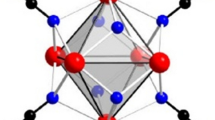
Some other dinitridocarbonate compounds appear with structures in which RE ions form octahedral RE6 arrangements such as in the oxygen centered RE6O cluster in RE8O(CN2)10Br2 (RE = La, Ce, Pr, Nd) [10]. The representative structure element of this isotypic series is the [RE6O(NCN)8] entity that can be well related to the [M6X8]-type cluster, which is based on an octahedral M6 cluster core with eight face capping X atoms, representing a most prominent motif in cluster chemistry (Fig. 1, left).
Other examples of [M6X8]-type arrangements are represented by [RE6N4(NCN)4] entities in structures of RE2N(CN2)Cl [11] (RE = La, Ce) and RE2N(CN2)I [12] (RE = La, Gd).
All these compounds appear as transparent crystal powders, obtained from typical SSM reactions at temperatures around 500–600 °C, with an example given in (1).
Rare earth carbodiimide nitrides RE2N(CN2)Cl with RE = La, Ce, and RE2N(CN2)I with RE = La, Gd were reported to show a structural pattern based on the linear chains of edge-bridging [RE6] octahedra. This pattern appears closely related to the arrangement of atoms in the structure of the metal-rich sesquichlorides RE2X3 (RE = Y, Gd, Tb; X = Cl, Br) [13,14,15,16] whose structures can be envisioned by condensing [M6X8] clusters through sharing trans-edges (Fig. 1, right) [17].
Rare earth sesquichlorides represent metal-rich compounds, having formally three excess electrons per formula unit RE2X3. Yttrium sesquichloride nitride, known as β-Y2Cl3N crystallizes isostructural to the binary compound (Fig. 2) and would represent a salt-balanced compound if there was no nitrogen deficiency assumed as β-Y2Cl3N1−x. A similar linear chain arrangement can be found in the structure of RE2N(CN2)Cl by substituting all face-capping Cl ligands in Y2Cl3 by (NCN)2− ions (Fig. 2, right).
In course of our exploration of rare earth dinitridocarbonates we have performed SSM reactions for different anion compositions and for potentially metal-rich systems. Herein we describe the discovery of a new isotypic series of compounds having the composition RE4O2(CN2)3X2, that represents another example for trans-edge bridging RE6-octahedra. The synthesis and crystal structure of RE4O2(CN2)3Cl2 compounds are reported for RE = Y, Sm, Gd, Tb.
Experimental Materials and Methods
Synthesis
All manipulations of the starting materials were performed in a glovebox under dry argon atmosphere.
Preparation of Li2(CN2) and REOCl
Li2(CN2) used in reactions was obtained by reacting Li3N (Alfa Aesar 99.4%) and C3N3(NH2)3 in 1:2 molar ratio. The mixture (total mass of approximately 6.9 g) was heated at 270 °C in an open corundum boat under flowing argon for 20 min. Afterwards, the temperature was raised to 330 °C with a rate of 0.5 °C/min and kept for 10 min. The mixture was then heated to 600 °C with a rate of 3.5 °C/min, kept at this temperature for 30 min, and then cooled to room temperature with the same rate. Afterwards, the produced material was transferred into a glove box (Ar). The purity of the material was verified by powder X-ray diffraction.
REOCl was prepared from RE2O3 (RE = Y (Rhone Poulence Spécialités Chemiques, 99.9 %), Sm (Ventron, 99.999%), Gd (Ventron, 99.999%), respectively Tb (Aldrich, 99.99 %)) and ammonium chloride (Alfa-Aesar, sublimed) in 1:2 molar ratio. The mixture was heated with a rate of 2 °C/min to 500 °C in an open corundum boat under argon flow, kept at this temperature for 2 h, and cooled to room temperature with a rate of 2 °C/min. The purity of the material was inspected by powder X-ray diffraction.
Synthesis of RE 4O2(CN2)3Cl2 (RE = Y, Sm, Gd, Tb)
All compounds were prepared following the same procedure: A mixture of REX3 (Sigma-Aldrich 99.99%), REOX and Li2(CN2) was ground in an agate mortar following a molar ratio of 2:2:3 (total mass ca. 200 mg). The mixture was loaded and fused into an evacuated silica ampoule, heated to 600 °C with a heating rate of 2 °C/min, kept at this temperature for 78 h, and then cooled to room temperature with a rate of 0.1 °C/min.
Cerium doped samples of Y4O2(CN2)3Cl2:Ce were prepared under the same conditions, wherein 5 mol% of the employed YCl3 were replaced by CeCl3.
Single-Crystal X-ray Diffraction
Single crystals of Y4O2(CN2)3Cl2 were mounted on a micro loop and placed onto a Rigaku XtaLAB Synergy-S single-crystal X-ray diffractometer equipped with a HyPix-6000HE detector and monochromated Mo–Kα radiation (λ = 71.073 pm). The X-ray intensities were corrected for absorption with numerical method. The structure was solved by direct methods with OLEX software [18]. Cooling was applied with a stream of nitrogen to T = 150(1) K using a cryostream cooler (series 800, Oxford Cryosystems) and maintained at this temperature during the measurement.
Powder X-ray Diffraction
Reaction products were inspected by powder X-ray diffraction (StadiP, Stoe, Darmstadt, Ge-monochromated Cu–Kα1 radiation) in the range 5<Θ<120°. The structure of REO2(CN2)3Cl2 (RE = Y, Gd, Sm, Tb) were refined using the Fullprof-Suite (Fig. 3) [19].
X-ray powder diffraction pattern of Tb4O2(CN2)3Cl2 with Rietveld refinement. The calculated pattern (black line) is superimposed with the observed pattern (marked by red circles). Green ticks mark the Bragg reflections, and the difference curve between the observed and calculated pattern is shown in the lower part of the graph (blue line) (color figure online)
Infrared Spectroscopy
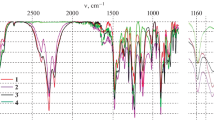
The samples were prepared in a KBr pellet and the infrared vibrational spectrum of Y4O2(CN2)3Cl2 was recorded with a Bruker Vertex 70 spectrometer within the range of 400–4000 cm−1.
Energy-Dispersive X-ray (EDX) Measurement
The EDX spectroscopy data were collected with a Hitachi SU8030 scanning electron microscope and a Bruker QUANTAX 6G EDX detector. Crystals of Y4O2(CN2)3Cl2 were fixed on a carbon tape.
Luminescence Spectroscopy
Emission spectra of Y4O2(CN2)3Cl2:Ce were recorded with a fluorescence spectrometer FLS920 (Edinburgh Instruments) equipped with a 450 W xenon arc lamp (OSRAM) and a sample chamber installed with a mirror optic for powder samples. For detection, a R2658P single-photon counting photomultiplier tube (Hamamatsu) was used. All luminescence spectra were recorded with a spectral resolution of 1 nm, a dwell time of 0.5 s in 1 nm steps, and three repeats.
Results and Discussion
Synthesis of RE 4O2(CN2)3 Cl 2
RE4O2(CN2)3Cl2 compounds were synthesized by solid-state metathesis reaction from appropriate mixtures of RECl3, Li2(CN2), and REOCl according to reaction (2) at 600 °C.
The reaction products of (2) appeared as crystalline powders of RE4O2(CN2)3Cl2 with the metathesis salt LiCl, as identified by powder XRD diffraction. Crystal powders of Y4O2(CN2)3Cl2 appear transparent, containing rod-shaped crystals (Fig. 4).
An energy-dispersive X-ray spectroscopy measurement (EDX) was performed to analyze the heavy atoms composition of a crystalline sample of Y4O2(CN2)3Cl2. For this purpose, crystalline material of Y4O2(CN2)3Cl2 was investigated with a scanning electron microscope (SEM) with a micrograph exemplified in Fig. 5. The heavy atom composition of Y4O2(CN2)3Cl2 was confirmed by averaging measurements at five spots of the crystalline material to yield a Y:Cl ratio of 3.97:2.0.
Crystal Structure of RE 4O2(CN2)3 Cl 2
The crystal structure of Y4O2(CN2)3Cl2 was solved and refined from single-crystal X-ray diffraction (XRD) data with the monoclinic space group C2/m, with selected refinement data given in Table 1. The series of RE4O2(CN2)3Cl2 compounds (RE = Sm, Gd, Tb) was assigned by isotypic indexing of the XRD powder patterns, with the refined lattice parameters given in Table 2.
The characteristic building block in the structure of Y4O2(CN2)3Cl2 is the [Y6O4(CN2)4] fragment (Fig. 6, top) that can be derived from the motif of a [M6X8]-type cluster, which is typically made up of six metal atoms (M) and eight chalcogenide or halide atoms (X). Within the given cluster nomenclature [17] these (X) ligands are assigned as inner (i) ligands, or as (i–i) ligand when there is a bridging connectivity between inner (or face-capping) positions of adjacent clusters, or as (i–a) ligands when the connectivity of an inner ligand is directed to the outer (a) (or apical) position of an adjacent cluster. Thereby, the (i–a) connectivity of the shared (NCN)2− ion converts itself into a (a–i) connectivity when looking from the position of an adjacent cluster.
Following this nomenclature, the connectivity of clusters in our title compound corresponds to [Y6O4(CN2)i–i2/2(CN2)i–a2/2], shown at the top of Fig. 6. The presence of i–aN(1)–C(1)–N(2)a–i bridges between clusters and of additional Cl- ions in the structure completes the connectivity pattern in the structure as [Y6O4(CN2)i–i2/2(CN2)i–a2/2(CN2)a–i2/2Cla–a8/2], shown at the bottom of Fig. 6, with all surrounding atoms that are connecting the cluster into a complex network structure.
A most remarkable feature in the crystal structure is the edge-connected \({}_{\infty }{}^{1}[{Y}_{4}{O}_{2}]\) cluster chain via basal Y2 atoms. When an isolated cluster is connected into a chain, the atoms Y2, O and Cl1 are shared between clusters to finally yield [Y2Y4/2O4/2(CN2)i–i2/2(CN2)i–a2/2(CN2)a–i2/2Cl8/2a–a]. A section of the corresponding chain structure is given in Fig. 7.
The structure of Y4O2(CN2)3Cl2 contains two distinct, nearly linear (NCN)2− ions. The (NCN)2− ions with the nonsymmetrici–a connectivity of N1–C1–N2 (and the oppositea–i) N2–C1–N1 can be viewed as cyanamides (dN-C = 116.8(9) pm and 104.5(9) pm), whereas the i–i connectivity of N3–C2–N3 corresponds to a (2/m) symmetric carbodiimide bridge (dN-C = 110.0(5) pm).
The structures of Y2Cl3 [16] and β-Y2Cl3N [20] are represented by an arrangement of yttrium octahedra which are, however, stretched along the chain direction, with the interatomic distances presented in Table 3. Y2Cl3 has been reported to be a semiconductor with an estimated band gap in the order of 1 eV, β-Y2Cl3N has been suggested to show a nitrogen deficiency. Both of them crystalize as black, shiny needles, which are difficult to handle because they fray into fibres when being cut [16, 20]. In spite of a quite similar structure, crystals of Y4O2(CN2)3Cl2 are transparent and thus represent a salt-balanced compound.
The presence of metal-to-metal bonding in Y2Cl3 and (β-)Y2Cl3N1−x in contrast to Y4O2(CN2)3Cl2 should make a difference for the distances, compiled for the Y–Y contacts of all these yttrium compounds in Table 3. However, the significance of interatomic metal-to-metal distances is not always easy to read, not only because heteroatomic bonding is considered much stronger than metal-to-metal bonding.
In Y2Cl3 and (β-)Y2Cl3N the octahedra are distinctly compressed along the shared edge and the Y2–Y2 distance of 326.6 pm is the shortest metal-to-metal distance observed in Y2C13. The presence of short metal-to-metal distances can be an indicator for effective bonding. Band structure calculations on Y2C13 [16] revealed that three d bands (of the Y4Cl6 unit cell content) are strongly metal-metal bonding along the shared Y–Y edge (herein denoted as Y2) and are split off from the block of remaining bands by a gap of about 0.7 eV.
A closer look at localized molecular orbitals of the system reveals three bonding orbitals for the total of six electrons of (Y2Cl3)2. Following this view, two electrons reside in a sigma bond (2c–2e bond) joining the basal metal atom pairs (Y2–Y2), and two time two electrons were described to reside in 4c–2e bond orbitals delocalized over rhombuses that have the basal bond (cluster connecting edge) at their intersection, as reported by Hughbanks [21]. Thus, in a localized picture, each uncapped triangular cluster face hosts one electron. This triangular cluster face remains uncapped once [M6X8] clusters are condensed into chains, as can be imagined from looking at Fig. 1 (at right). It creates tetrahedral voids that are occupied by a nitride ion in β-Y2Cl3N (Fig. 2, left). In the structure of the cluster chain of Y6-octahedra in Y4O2(CN2)3Cl2 these tetrahedral voids are occupied by oxide ions with Y–O distances ranging between 226 and 231 pm. The Y–Y bond lengths in the structure of Y4O2(CN2)3Cl2 are generally more regular with only a slight contraction of the shared Y2–Y2 edge of 355.3 pm. The connectivity of the cluster chains in structure of Y4O2(CN2)3Cl2 is projected in Fig. 8, showing the complete arrangement in the unit cell.
In general, the notation as cluster for the given rare earth compounds is different when compared to some transition-metal cluster compounds. The term “cluster” was originally used for the description of compounds containing metal atoms connected by metal-to-metal bonds [22]. Today, the term is also used for compounds compromising an ensemble of metal atoms that are interconnected through bridging ligands. Sometimes the term “coordination cluster” is used [23].
Moreover, the given comparison of the salt-like title compound with Y2Cl3 does not show substantial differences in the overall arrangement pattern of yttrium atoms in the structures. In this respect, it should be recalled, that connecting lines drawn between metal atoms in the given figures just represent guidelines for the eye.
Infrared Studies
Two distinct [NCN]2- ions are identified in the crystal structure of Y4O2(CN2)3Cl2. The connectives between adjacent cluster chains are accomplished by symmetrical N3–C2–N3 bridges along the c-axis displayed as (symmetrical,i–i) carbodiimide bridges. These distances amount to dN-C = 110.0(5) pm. The cyanamide bridges (i–a, a–i) of N1–C1–N2 are non-symmetrical with dN-C = 116.8(9) pm and 104.5(9) pm. The total proportion of these bridges in the structure of Y4O2(CN2)3Cl2 is 1:2. The infrared spectrum recorded for Y4O2(CN2)3Cl2 shows typical features of the presence of [NCN]2- ions (Fig. 9). The asymmetric stretch is split into two signals within the given resolution of our spectrometer, centered at 2154 cm−1 and 2099 cm−1 cm−1. The bending modes are obtained between 663 and 627 cm−1 [24, 25].
Luminescence of Y4O2(CN2)3Cl2:(Ce3+)
The emission spectrum of the compound doped with 5 mol% cerium w.r.t yttrium under excitation at 340 nm is shown in Fig. 10.
The luminescence spectrum shows a broad emission band with a maximum at 550 nm and a half-width of 135 nm. This emission is ascribed to the transitions from [Xe]5d1 state to the [Xe]4f1 (2F5/2 and 2F7/2) ground state of Ce3+. Such a large width is typical for Ce3+ luminescence due to the ground state splitting of the [Xe]4f1 configuration by spin-orbit coupling [26].
Conclusions
A series of new rare earth dinitridocarbonate compounds was prepared by means of solid-state metathesis reactions with have been established as a powerful tool for the synthesis for this class of compounds. The isotypic compounds RE4O2(CN2)3Cl2 with RE = Y, Sm, Gd and Tb resemble structures closely related to that of Y2Cl3, appearing with a structure based on edge-shared one-dimensional RE6-cluster chains; thereby allowing a glimpse on the difference of structural and electronic details, such as the metal-to-metal contacts of electron-rich (semiconducting) Y2Cl3 versus salt-balanced RE4O2(CN2)3Cl2. Based on the localized bonding picture for Y2Cl3 it can be understood that the bridging edge of cluster chains is a most sensitive indicator for the presence of metal-to-metal bonding as can be read from the series of Y4O2(CN2)3Cl2, β-Y2Cl3N, Y2Cl3. Localized bonding in triangular cluster faces, as reported for Y2Cl3, cannot be present once face-capping oxygen appear, as given in the structure of Y4O2(CN2)3Cl2.
The series of RE4O2(CN2)3X2 and formerly developed materials such as RE4O2(CN2)3Cl2 and RE2N(CN2)X (X = halide) demonstrate the versatility of the [M6X8]-type structure, wherein X can stand for various anion types. So far, all rare earth compounds that were developed with (NCN)2− ions have been salt-like compounds, but metal-rich compounds may be at hand as well. Salt balanced compounds are of potential for optical properties, especially for their photoluminescence properties.
Data Availability
One Supplementary file.
References
M. Neukirch, S. Tragl, and H. J. Meyer (2006). Inorg. Chem. 45, 8188–8193.
H.-J. Meyer (2010). Dalton Trans. 39, 5973–5982.
M. G. Down, M. J. Haley, P. Hubberstey, R. J. Pulham, and A. E. Thunder (1978). J Chem Soc Dalton Trans. https://doi.org/10.1039/dt9780001407.
P. Kallenbach, M. Ströbele, and H.-J. Meyer (2020). Z. Anorg. Allg. Chem. 646, 1281–1284.
R. Srinivasan, J. Glaser, S. Tragl, and H.-J. Meyer (2005). Z. Anorg. Allg. Chem. 631, 479–483.
R. Srinivasan, S. Tragl, and H.-J. Meyer (2005). Z. Anorg. Allg. Chem. 631, 719–722.
H. Yasuhiro, T. Masao, K. Shinichi, and K. Fumikazu (1994). Chem. Lett. 23, 1963–1966.
L. Unverfehrt, M. Ströbele, J. Glaser, and H.-J. Meyer (2009). Z. Anorg. Allg. Chem. 635, 1947–1952.
Y.-C. Wu, T.-M. Chen, C.-H. Chiu, and C.-N. Mo (2010). J. Electrochem. Soc. 157, J342.
A. Siai, C.-D. Brand, M. Ströbele, D. Enseling, T. Jüstel, and H.-J. Meyer (2022). J. Clust. Sci. 34, 1001–1008.
R. Srinivasan, M. Ströbele, and H.-J. Meyer (2003). Inorg. Chem. 42, 3406–3411.
D. Dutczak, A. Siai, M. Ströbele, D. Enseling, T. Jüstel, and H.-J. Meyer (2020). Eur. J. Inorg. Chem. 2020, 3954–3958.
A. Simon, N. Holzer, and H. Mattausch (1979). Z. Anorg. Allg. Chem. 456, 207–216.
D. A. Lokken and J. D. Corbett (1973). Inorg. Chem. 12, 556–559.
R. E. Araujo and J. D. Corbett (1981). Inorg. Chem. 20, 3082–3086.
H. Mattausch, J. B. Hendricks, R. Eger, J. D. Corbett, and A. Simon (1980). Inorg. Chem. 19, 2128–2132.
H. Schäfer and H. G. Schnering (1964). Angew. Chem. 76, 833–868.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann (2009). J. Appl. Crystallogr. 42, 339–341.
J. Rodríguez-Carvajal (1993). Physica B Condens. Matter 192, 55–69.
H.-J. Meyer, N. L. Jones, and J. D. Corbett (1989). Inorg. Chem. 28, 2635–2637.
K. A. Yee and T. Hughbanks (1992). Inorg. Chem. 31, 1620–1625.
F. A. Cotton, N. F. Curtis, C. B. Harris, B. F. G. Johnson, S. J. Lippard, J. T. Mague, W. R. Robinson, and J. S. Wood (1964). Science 145, 1305–1307.
A. T. Wagner and P. W. Roesky (2016). Eur. J. Inorg. Chem. 2016, 782–791.
G. Rapi and G. Sbrana (1971). J. Am. Chem. Soc. 93, 5213–5217.
S. T. King and J. H. Strope (1971). J. Chem. Phys. 54, 1289–1295.
D. J. Robbins (1979). J. Electrochem. Soc. 126, 1550–1555.
Acknowledgements
Funding by the Deutsche Forschungsgemeinschaft (DFG) through grant ME 914/34-1 is gratefully acknowledged. The authors thank Ms. Elke Nadler (University Tübingen) for recording SEM images and EDX data.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Author information
Authors and Affiliations
Contributions
C-DB did the syntheses, MS solved and refined the crystals structures, DE performed luminescence measurements, TJ and H-JM supervised the research and wrote/revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Not applicable.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brand, CD., Ströbele, M., Enseling, D. et al. Y4O2(CN2)3Cl2, Its Relation to the Metal-Rich Y2Cl3, and the Photoluminescence of Y4O2(CN2)3Cl2:Ce. J Clust Sci 35, 101–107 (2024). https://doi.org/10.1007/s10876-023-02463-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02463-2