Abstract
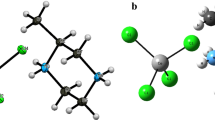
An original vanadium (V) oxyfluoride, containing a polymeric [(H2C4O4)VO2F] 2n−n chains and diethylammonium cations, has been synthesized by slow evaporation from aqueous solutions and characterized by single-crystal X-ray diffraction and vibrational spectroscopies (IR and Raman). (Hdea)2[(H2C4O4)VO2F]·H2O (dea: diethylamine) crystallizes in the monoclinic system, space group C2/c. The structure can be described as a succession of equivalent layers perpendicular to b. The anionic [(H2C4O4)VO2F] 2n−n polymeric chain is composed of trigonal bipyramidal VO4F polyhedra and fumaric acid (H2C4O4) groups sharing O2 corners. The cohesion of the structure is provided by a network hydrogen-bonding. The IR and Raman spectra exhibit characteristic bands of all groups present in the structure. Additionally UV–Vis diffuse reflectance spectrum was recorded in order to investigate the band gap nature. The measurements show that this compound exhibit semiconducting behavior with an optical band gap of 3.51 eV.



Similar content being viewed by others
References
A. Butler, M. J. Clague, and G. E. Meister (1994). Chem. Rev. 94, 625–638.
K. H. Thompson, J. H. McNeil, and C. Orvig (1999). Chem. Rev. 99, 2561–2572.
D. C. Crans (2000). J. Inorg. Biochem. 80, 123–131.
D. C. Crans, J. J. Smee, E. Gaidamauskas, and L. Yang (2004). Chem. Rev. 104, 849–902.
C. N. Caughlan, H. M. Smith, and K. Watenpaugh (1966). Inorg. Chem. 5, 2131–2134.
W. Priebsch and D. Rehder (1990). Inorg. Chem. 29, 3013–3019.
D. C. Crans, R. W. Marshman, M. S. Gottlieb, O. P. Anderson, and M. M. Miller (1992). Inorg. Chem. 31, 4939–4949.
K. Waltersson (1979). J. Solid. State. Chem. 28, 121–131.
T. Mahenthirarajah, Y. Li, and P. Lightfoot (2008). Inorg. Chem. 47, 9097–9102.
F. Himeur, P. K. Allan, S. J. Teat, R. J. Goff, R. E. Morris, and P. Lightfoot (2010). Dalton. Trans. 39, 6018–6020.
F. H. Aidoudi, D. W. Aldous, R. J. Goff, M. Z. Slawin Alexandra, J. P. Attfield, R. E. Morris, and P. Lightfoot (2011). Nat. Chem. 3, 801–806.
M. D. Donakowski, R. Gautier, J. Yeon, D. T. Moore, J. C. Nino, P. S. Halasyamani, and K. R. Poeppelmeier (2012). J. Am. Chem. Soc. 134, 7679–7689.
D. W. Aldous, A. M. Z. Slawin, and P. Lightfoot (2008). J. Solid. State. Chem. 181, 3033–3036.
D. W. Aldous, N. F. Stephens, P. Lightfoot (2007) Dalton Trans. 2007, 4207–4213. doi:10.1039/B708889B.
D. W. Aldous, N. F. Stephens, P. Lightfoot (2007) Dalton Trans. 2007, 2271–2282. doi:10.1039/b702146a.
N. F. Stephens, M. Buck, and P. Lightfoot (2005). J. Mater. Chem. 15, 4298–4300.
F. H. Aidoudi, C. Black, K. S. A. Arachchige, M. Z. S. Alexandra, R. E. Morris, and P. Lightfoot (2014). Dalton Trans. 43, 568–575.
F. H. Aidoudi, P. J. Byrne, P. K. Allan, S. J. Teat, P. Lightfoot, and R. E. Morris (2011). Dalton Trans. 40, 4324–4331.
L. Clark, J. C. Orain, F. Bert, M. A. De Vries, F. H. Aidoudi, R. E. Morris, P. Lightfoot, J. S. Lord, M. T. F. Telling, P. Bonville, J. P. Attfield, P. Mendels, and A. Harisson (2013). Phys. Rev. Lett. 110, 207208.
D. W. Aldous, N. F. Stephens, and P. Lightfoot (2007). Inorg. Chem. 46, 3996–4001.
S. Rostamzadehmansor, G. Ebrahimzadehrajaei, S. Ghammamy, K. Mehrani, and L. Saghatforoush (2008). J. Fluor. Chem. 129, 674–679.
P. DeBurgomaster, W. Ouellette, H. Liu, C. J. O’Connor, G. T. Yee, and J. Zubieta (2010). Inorg. Chim. Acta. 363, 1102–1113.
R. C. Haushalter, L. M. Meyer, and J. Zubieta in M. H. Chisholm (ed.), Early Transition Metal Clusters with π-Donor Ligands (VCH Publishers, New York, 1995), pp. 217–246.
D. J. Chesnut, D. Hagrman, P. J. Zapf, R. P. Hammond, R. L. Laduca, R. C. Haushalter, and J. Zubieta (1999). Coord. Chem. Rev. 190–192, 737.
R. C. Finn, J. Zubieta, and R. C. Haushalter (2003). Prog. Inorg. Chem. 51, 421.
M. I. Khan, Q. Chen, H. Höpe, S. Parkin, C. J. O’Connor, and J. Zubieta (1993). Inorg. Chem. 32, 2929–2937.
C. Ninclaus, D. Riou and G. Férev (1997) Chem. Commun. 851–852. 1997, doi:10.1039/A607863J.
A. Müller, R. Rohlfing, A.-L. Barra, and D. Gatteschi (1993). Adv. Mater. 5, 915–917.
A. Müller, J. Meyer, H. Bögge, A. Stammlerand, and A. Botar (1998). Chem. Eur. J. 4, 1388–1397.
S. Ahmad, A. A. Isab, S. Ali, and A. R. Al-Arfaj (2006). Polyhedron. 25, 1633–1645.
M. D. Smith, S. M. Blau, K. B. Chang, T. T. Tran, M. Zeller, P. S. Halasyamani, J. Schrier, and A. J. Norquist (2012). J. Solid. State. Chem. 195, 86–93. doi:10.1016/j.jssc.2012.02.024.
M. Aureliano and D. C. Crans (2009). J. Inorg. Biochem. 103, 536–546. doi:10.1016/j.jinorgbio.2008.11.010.
A. Sarkar and S. Pal (2008). Polyhedron. 27, 3472–3476. doi:10.1016/j.poly.2008.08.001.
V. W. Day, W. G. Klemperer, and O. M. Yaghi (1989). J. Am. Chem. Soc. 111, 4518–4519.
V. W. Day, W. G. Klemperer, and O. M. Yaghi (1989). J. Am. Chem. Soc. 111, 5959–5961.
D. Hou, K. S. Hagen, and C. L. Hill (1992). J. Am. Chem. Soc. 114, 5864–5866.
D. Hou, K. S. Hagen, C. L. Hill (1993) J. Chem. Soc Chem. Commun. 426–428. doi: 10.1039/C39930000426.
G. A. Senchyk, V. O. Bukhan’ko, A. B. Lysenko, H. Krautscheid, E. B. Rusanov, A. N. Chernega, M. Karbowiak, and K. V. Domasevitch (2012). Inorg. Chem. 51, (15), 8025–8033. doi:10.1021/ic3000894.
Z. Bircsak and W. T. A. Harrison (1998). J. Solid. State. Chem. 140, 272–277.
Y.-M. Cui, Y.-J. Cai, and W. Chem (2011). Synth. React. Inorg. Met.-org. Chem. 41, 1244–1248. doi:10.1080/15533174.2011.591877.
M. Padmanabhan, J. C. Joseph, A. Thirumurugan, C. N. R. Rao (2008) Dalton. Trans. 2809–2811. doi: 10.1039/b718623a (and references therein).
J. Song, B.-C. Wang, H.-M. Hu, L. Gou, Q.-R. Wu, X.-L. Yang, Y.-Q. Shangguan, F.-X. Dong, and G.-L. Xue (2011). Inorg. Chim. Acta. 366, 134–140. doi:10.1016/j.ica.2010.10.020.
K N. Lazarou, A. Terzis, S P. Perlepes, C P. Raptopoulou (2010) Polyhedron. 29, 46–53. doi: 10.1016/j.poly.2009.05.075.
J. Do, Y. Lee, J. Kang, and A. J. Jacobson (2012). Inorg. Chim. Acta. 382, 191–194. doi:10.1016/j.ica.2011.11.061.
W. Gong, H. Niu, J. Zhang, J. Song, C. Mao, and S. Zhang (2014). Inorg. Chim. Acta. 418, 93–98. doi:10.1016/j.ica.2014.04.009.
D. M. Young, U. Geiser, A. J. Schultz, and H. H. Wang (1998). J. Am. Chem. Soc. 120, 1331.
K. Seki, S. Takamizawa, W. Mori (2001) Chem. Lett. 30(2), 122–123. doi:10.1246/cl.2001.122.
S. Konar, E. Zangrando, and N. R. Chaudhuri (2003). Inorg. Chim. Acta. 355, 264.
Y. Zheng and H. Xie (2004). J. Solid. State. Chem. 177, 1352.
D. Ghoshal, G. Mostafa, T. K. Maji, E. Zangrando, T. Lu, J. Ribas, and N. R. Chaudhuri (2004). New J. Chem. 28, 1204.
N. P. Porollo, Z. G. Aliev, G. I. Dzhardimalieva, I. N. Ivleva, I. E. Uflyand, A. D. Pomogailo, and N. S. Ovanesyan (1997). Russ. Chem. Bull. 46, 362.
E. Pajtasova, E. Jona, M. Koman, and D. Ondrusova (2001). Pol. J. Chem. 75, 1209.
A. Y. Robin and K. M. Fromm (2006). Coord. Chem. Rev. 250, 2127.
Y. Zhou, M. Hong, X. Wu (2006) Chem. Commun. 135–143. doi: 10.1039/B509458P.
C. Coulon, H. Miyasaka, and R. Clérac (2003). Struct. Bond. 122, 163.
D-K. Bucar, G.S. Papaefstathiou, T.D. Hamilton, Q.L. Chu, I.G. Georgiev, L.R. MacGillivray (2007) Eur. J. Inorg. Chem. 2007, 4559–4568. doi:10.1002/ejic.200700442.
J. Y. Lee, O. K. Fartha, J. Roberts, K. A. Scheidt, S. T. Nguyen, and J. T. Hupp (2009). Chem. Soc. Rev. 38, 1450.
L. Ma, C. Abney, and W. Lin (2009). Chem. Soc. Rev. 38, 1248.
M. Kurmoo (2009). Chem. Soc. Rev. 38, 1353.
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk (2009). Chem. Soc. Rev. 38, 1330.
L. J. Murray, M. Dincӑ, and J. R. Long (2009). Chem. Soc. Rev. 38, 1294.
G. K. H. Shimizu, R. Vaidhyanathan, and J. M. Taylor (2009). Chem. Soc. Rev. 38, 1430.
C. P. Raptopoulou, V. Tangoulis, and E. Devlin (2002). Angew. Chem. Int. Ed. 41, 2386.
A. K. Boudalis, C. P. Raptopoulou, B. Abarca, R. Ballesteros, M. Chadlaoui, J.-P. Tuchagues, and A. Terzis (2006). Angew. Chem. Int. Ed. 45, 432.
A.K. Boudalis, C.P. Raptopoulou, V. Psycharis, Y. Sanakis, B. Abarca, R. Ballesteros, M. Chadlaoui (2007) Dalton. Trans. 2007, 3582–3589. doi:10.1039/B705962K.
A.K. Boudalis, C.P. Raptopoulou, V. Psycharis, B. Abarca, R. Ballesteros (2008) Eur. J. Inorg, Chem. 2008, 3796–3801. doi:10.1002/ejic.200800400.
A. K. Boudalis, M. Pissas, C. P. Raptopoulou, V. Psycharis, B. Abarca, and R. Ballesteros (2008). Inorg. Chem. 47, 10674.
A. N. Georgopoulou, C. P. Raptopoulou, V. Psycharis, R. Ballesteros, B. Abarca, and A. K. Boudalis (2009). Inorg. Chem. 48, 3167.
Th.C Stamatatos, D. Foguet-Albiol, S.-C. Lee, C.C. Stoumpos, C.P. Raptopoulou, A. Terzis, W. Werndofer, S.P. Perlepes, G. Christou (2007) J. Am. Chem. Soc. 129, 9484.
Bruker APEX2 and SAINT (Bruker AXS Inc., Madison, 2009).
A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, and R. Spagna (1999). J. Appl. Crystallogr 32, 115–119.
G. Sheldrick (2008). Acta. Cryst A64, 112–122.
L. J. Farrugia (1999). J. Appl. Crystallogr. 32, 837–838.
A. L. Spek Utrecht University (Utrecht, The Netherlands, 2001).
K. Brandenburg DIAMOND 2.0, Visual Crystal Structure Information System (Crystal impact Gbr, Bonn, 2007).
I. D. Brown (1976). Acta Cryst. A32, 24–31. doi:10.1107/S0567739476000041.
Addison. A. W, Rao. T. N, Reedijk. J, Van Rijn. J, Verschoor. G. C (1984) J. Chem. Soc. Dalton. Trans. 1349–1356.
I. D. Brown (1992). Acta. Cryst. B48, 553–572.
N. E. Bresse and M. O’Keeffe (1991). Acta. Cryst. B47, 192–197.
I. Omri, M. Graia, and T. Mhiri (2014). J. Clust. Sci.. doi:10.1007/s10876-014-0768-3.
P. DeBurgomaster and J. Zubieta (2010). Acta. Cryst. E66, m1303.
N. Buchholz, M. Leimkühler, L. Kiriazis, and R. Mattes (1988). Inorg. Chem. 27, 2035–2039.
E. Bozkurt, İ. Uçar, İ. Kartal, A. Bulut, and O. Büyükgüngör (2008). J. Phys. Chem. Solids. 69, 2109–2115. doi:10.1016/j.jpcs.2008.03.011.
J. Kang, Y. Yang, S. Pan, H. Yu, and Z. Zhou (2014). J. Mol. Struct. 1056–1057, 79–83. doi:10.1016/j.molstruc.2013.10.009.
T. Sivakumar, H. Y. Chang, J. Baek, and P. S. Halasyamani (2007). Chem. Mater. 19, (19), 4710–4715. doi:10.1021/cm071188p.
A. Grzechnik and P. F. McMillan (1995). J. Phys. Chem. Solids 56, 159–164.
H. Nefzi, F. Sediri, H. Hamzoui, and N. Gharbi (2012). J. Solid. State. Chem. 190, 150–156. doi:10.1016/j.jssc.2012.02.013.
Y.-T. Li, C.-Y. Zhu, Z.-Y. Wu, M. Jiang, and C.-W. Yan (2010). Transit. Met. Chem. 35, 597–603. doi:10.1007/s11243-010-9369-7.
J. Chrappová, P. Schwendt, and J. Marek (2005). J. Fluor. Chem. 126, 1297–1302. doi:10.1016/j.jfluchem.2005.06.009.
L. Mai and C. Han (2008). Mater. Lett. 62, 1458–1461. doi:10.1016/j.matlet.2007.08.088.
N. V. Venkataraman, S. Bhagyalakshmi, S. Vasudevan, and R. Seshadri (2002). Phys. Chem. Chem. Phys. 4, 4533–4538. doi:10.1039/b204983j.
J. L. Castro, M. R. López-Ramírez, J. F. Arenas, and J. C. Otero (2005). Vib. Spectrosc. 39, 240–243. doi:10.1016/j.vibspec.2005.04.007.
Z.-H. Li and H.-D. Bai (2008). J. Zhejiang. Univ. Sci A. 9, (1), 143–148. doi:10.1631/jzus.A071180.
Y. Xia, P. Wu, Y. Wei, Y. Wang, and H. Guo (2006). Cryst. Growth Des. 6, (1), 253–257.
J.-H. Liao, J.-S. Juang, and Y.-C. Lai (2006). Cryst. Growth Des. 6, (2), 354–356.
Elizabeth E. Chain (1991). Appl. Opt. 30, (19), 2782–2787.
M. Benmoussa, E. Ibnouelghazi, A. Bennouna, and E. L. Ameziane (1995). Thin. Solid. Films. 265, 22–28.
Acknowledgments
The crystal data collection of the title compound was done in the “Department of Chemistry, Faculty of Sciences of Sfax, University of Sfax, BP 1171, 3038 Sfax, Tunisia”. We are grateful to Abdelhamid Ben Salah who supervised this experiment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Omri, I., Mhiri, T. & Graia, M. A New Vanadium (V) Coordination Based on [(H2C4O4)VO2F] 2n−n Polymeric Chains and Diethylammonium Cations, Synthesis, Crystal Structure, Vibrational and Optical Properties. J Clust Sci 26, 1267–1278 (2015). https://doi.org/10.1007/s10876-014-0811-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0811-4