Abstract
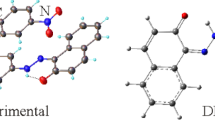
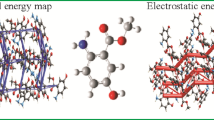
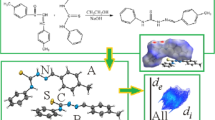
The presented study describe the crystal structure of 2-(Ethoxymethylene)malononitrile (1), C6H6N2O, in the monoclinic space group P21/m with Z = 2, a = 6.798(3), b = 6.172(3), c = 8.844(5) Å. The unit cell of a single crystal of 1 contains two antiparallel oriented molecules. Ethyl fragment demonstrates a disorder with equal occupancy values of 0.5 and a total site-occupation factor (s.o.f.) of 1.0. The molecules of 1 are linked into infinite chains of co-oriented molecules parallel to the a axis via N-H···N ≡ C close contacts with the distance of 2.494(3) Å. There are also weak hydrogen bonds > O···H- between the oxygen atom and the ethyl moiety. The estimation of the energy of non-covalent interactions was conducted by DFT method with different functionals. The best reproducibility of the geometric parameters of those interactions was obtained by using M06-2X functional. The estimated energy value was found to be − 1.20 kcal/mol.
Graphical Abstract






Similar content being viewed by others
Data Availability
The structure of crystal 1 has been deposited in the Cambridge Crystallographic Data Centre with deposition number CCDC 2180335. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033).
References
Diels O, Gärtner H, Kaack R (1922) Über Versuche Zur Darstellung Des carbonylcyanids und eine Methode Zur Gewinnung ungesättigter Amino-Säuren. Ber Dtsch Chem Ges A/B 55:3439–3448. https://doi.org/10.1002/cber.19220551013
Ochiai M, Yamamoto S, Suefuji T, Chen D-W (2001) Stereoselective synthesis of (Z)-Enethiols and their derivatives: Vinylic SN2 reaction of (E)-Alkenyl(phenyl)-λ3-iodanes with Thioamides. Org Lett 3:2753–2756. https://doi.org/10.1021/ol016356c
Konakahara T, Sugama N, Yamada A, Kakehi A, Sakai N (2001) Cyclization reaction of N-Silyl-1-azaallyl anions with Michael Acceptors as a New Synthetic Method of 2,3,5,6-Tetra- and 2,3,6-Trisubstituted pyridines. Heterocycles 55:313–322. https://doi.org/10.3987/COM-00-9096
Osipov AK, Anis’kov AA, Yegorova AY (2017) Synthesis and configuration of (arylamino)methylidene-3H-furan-2-ones. Russ J Org Chem 53:210–214. https://doi.org/10.1134/S1070428017020117
Sultana S, Kumar G, Sarma LS, Venkatramu V, Gangi Reddy NC (2023) Nitrogen-Doped TiO2 Nanotubes‐Catalyzed synthesis of small D‐π‐A‐Type knoevenagel adducts and β‐Enaminones. Eur J Org Chem 26:e202300032. https://doi.org/10.1002/ejoc.202300032
Gately TJ, Cook C, Almuzarie R, Islam I, Gardner Z, Iuliucci RJ, Al-Kaysi RO, Beran GJO, Bardeen CJ (2022) Effect of Fluorination on the polymorphism and Photomechanical properties of Cinnamalmalononitrile crystals. Cryst Growth Des 22:7298–7307. https://doi.org/10.1021/acs.cgd.2c00930
Huang H, Xu L (2020) Crystal structure of (E)-2-(5,5-dimethyl-3-(4-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)oxy)styryl)cyclohex-2-en-1-ylidene)malononitrile, C 25 H 19 N 5 O 4. Zeitschrift für Kristallographie -. New Cryst Struct 235:1073–1075. https://doi.org/10.1515/ncrs-2020-0176
Sun C-T, Li Q (2023) Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4. Zeitschrift für Kristallographie -. New Cryst Struct 238:1103–1104. https://doi.org/10.1515/ncrs-2023-0340
De Souza JM, Abdiaj I, Chen J, Hanson K, De Oliveira KT, McQuade DT (2020) Increasing scope of clickable fluorophores: Electrophilic Substitution of Ylidenemalononitriles. J Org Chem 85:11822–11834. https://doi.org/10.1021/acs.joc.0c01551
Belahlou H, Waszkowska K, Bouraiou A, Bendeif E, Taboukhat S, Bouchouit K, Sahraoui B (2020) New architecture of organo electronic chalcones derivatives: synthesis, crystal structures and optical properties. Opt Mater 108:110188. https://doi.org/10.1016/j.optmat.2020.110188
Rietsch P, Witte F, Sobottka S, Germer G, Krappe A, Güttler A, Sarkar B, Paulus B, Resch-Genger U, Eigler S (2019) Diaminodicyanoquinones: fluorescent dyes with high dipole moments and Electron‐Acceptor Properties. Angew Chem Int Ed 58:8235–8239. https://doi.org/10.1002/anie.201903204
Sysoiev D, Huhn T (2020) Basic enemies of photochromism: irreversible transformation of fluorinated diarylethenes to polyenic enamines and enols. Photochem Photobiol Sci 19:1511–1516. https://doi.org/10.1039/d0pp00292e
Menekse K, Chen P, Mahlmeister B, Anhalt O, Kudzus A, Stolte M, Würthner F (2020) Quinoidal dicyanomethylene-endcapped cyclopentadithiophenes as vacuum-processable n-type semiconductors. J Mater Chem C 8:15303–15311. https://doi.org/10.1039/D0TC02988B
Gräßler N, Wolf S, Holzmüller F, Zeika O, Vandewal K, Leo K (2019) Heteroquinoid Merocyanine dyes with High Thermal Stability as Absorber materials in Vacuum-Processed Organic Solar cells. Eur J Org Chem 2019:845–851. https://doi.org/10.1002/ejoc.201801512
Al-Refai M, Ali BF, Said AB, Geyer A, Marsch M, Harms K (2019) Synthesis, characterization, crystal structure and supramolecularity of ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate and a new polymorph of ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate. Acta Crystallogr E Cryst Commun 75:1357–1361. https://doi.org/10.1107/S2056989019011435
Kalkhambkar RG, Gayathri D, Gupta VK, Kant R, Jeong YT (2012) (E)-Ethyl 2-cyano-3-(furan-2-yl)acrylate. Acta Crystallogr E Struct Rep Online 68:o1482–o1482. https://doi.org/10.1107/S1600536812016510
Ding R, He Y, Xu J, Liu H, Wang X, Feng M, Qi C, Zhang J, Peng C (2012) Preparation and bioevaluation of 99mTc nitrido radiopharmaceuticals with pyrazolo[1,5-a]pyrimidine as tumor imaging agents. Med Chem Res 21:523–530. https://doi.org/10.1007/s00044-011-9558-8
CrysAlisPro A, Technologies Version 1.171.37.33 (release 27-03-2014 CrysAlis171.NET)
Bourhis LJ, Dolomanov OV, Gildea RJ, Howard JAK, Puschmann H (2015) The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment – Olex2 dissected. Acta Crystallogr Found Adv 71:59–75. https://doi.org/10.1107/S2053273314022207
Sheldrick GM (2015) SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr Found Adv 71:3–8. https://doi.org/10.1107/S2053273314026370
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J (2010) Fox DJ Gaussian 09, Rev. C.01,. Gaussian, Inc., Wallingford CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Zhao Y, Truhlar DG (2004) Hybrid Meta Density Functional Theory methods for Thermochemistry, Thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and Comparative Assessments for Hydrogen Bonding and Van Der Waals interactions. J Phys Chem A 108:6908–6918. https://doi.org/10.1021/jp048147q
Remya K, Suresh CH (2013) Which density functional is close to CCSD accuracy to describe geometry and interaction energy of small noncovalent dimers? A benchmark study using Gaussian09. J Comput Chem 34:1341–1353. https://doi.org/10.1002/jcc.23263
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Jones RG (1952) Reactions of orthoesters with active Methylene compounds. J Am Chem Soc 74:4889–4891. https://doi.org/10.1021/ja01139a046
Post HW, Erickson ER (1937) The reactions of Ortho Esters with certain acid anhydrides *. J Org Chem 02:260–266. https://doi.org/10.1021/jo01226a008
Castro Agudelo B, Cárdenas JC, Macías MA, Ochoa-Puentes C, Sierra CA (2017) Crystal structure of ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate: two conformers forming a discrete disorder. Acta Crystallogr E Cryst Commun 73:1287–1289. https://doi.org/10.1107/S2056989017010799
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theoret Chim Acta 44:129–138. https://doi.org/10.1007/BF00549096
Acknowledgements
This work was supported by the Russian Science Foundation (grant no. 24-23-00482 to VSG).
Author information
Authors and Affiliations
Contributions
V.S.G. and I.A.D. wrote the main manuscript text, A.E.S. and M.V.D. prepared Figs. 1, 2, 3 and 4. A.Yu.Ye. supervised the work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grinev, V.S., Demeshko, I.A., Sklyar, A.E. et al. Crystal Structure of 2-(Ethoxymethylene)Malononitrile, Hirshfeld Surface Analysis and DFT Evaluation of the Non-covalent Interactions Energy. J Chem Crystallogr (2024). https://doi.org/10.1007/s10870-024-01019-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10870-024-01019-0