Abstract
The crystal structure of [Cu3(C7H5O3)4(C20H20NO5)2(H2O)2]·2(H2O) (1) and analysis of temperature and field dependence of magnetic susceptibility is reported in this work. The structure of 1 is composed of trinuclear complex units and water molecules. The middle copper atom occupies the center of symmetry. N, O-bonded (6,7-dimethoxy-isoquinolin-1-yl)-(3,4-dimethoxy-phenyl)-methanolato ligands, 2-hydroxybenzoates with bridging carboxylic groups, and oxo-bridged water molecules connect the middle Cu(II) atom with the terminal copper atoms. Two 2-hydroxybenzoates coordinate the terminal copper atoms via one carboxylic oxygen and an O atom of the hydroxyl group. The analysis of copper coordination by bond-valence sum approach and relevant structural correlation is consistent with hexacoordinated Cu(II) centers. Cu···Cu separation is 3.0269(3) Å. The magnetism of 1 shows a strong ferromagnetic interaction between the neighboring metallic centers accompanied by very weak antiferromagnetic intermolecular interactions. The complex units are mutually held by π···π stack interactions of 2-hydroxybenzoates and hydrogen bonds.
Graphical Abstract
A new N,O bonded ligands, (R, S)-[(6,7-dimethoxy-isoquinolin-1-yl)-(3,4-dimethoxy-phenyl)-methanolate] coordinate the terminal atoms of the trinuclear copper(II) complex.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Structurally Closely Related Alkaloids to Papaverine
(1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxyisoquinoline) are important pharmacological drugs and biologically active compounds [1,2,3]. The molecule of papaverine (Scheme 1) is unstable toward oxygen and can be oxidized easily to papaverinol and papaveraldine [4,5,6,7]. However, the crystal structure of papaverine hydrochloride [8] is composed of relatively stable nonoxidized papaverine molecules. The formation of copper complexes with the potential ligands contained in human organisms can cause many changes in cells [9]. To our knowledge, until now, with papaverine, only the crystal structure of the binuclear complex [Zn2(C6H5COO)4(pap)2] (pap = papaverine) was resolved [10]. Carboxylic copper(II) complexes may be mononuclear, binuclear, tetranuclear, or polynuclear [11,12,13,14].
Chemical coordination [15] defined by Werner [16] is an array of atoms surrounding the central atom because of chemical bonding. In transition element complexes, the atoms often interact with the central atom very weakly. In the quantum chemical definition, atomic valence is a measure of the degree of electron sharing of the given atom with the other atoms [17]. According to the residual valency of an atom, the atoms in a molecule may be subvalent, normal, or hypervalent [18]. Such classification deviates from the traditional valence of an atom in the crystal structure. It suggests using the atomic valence approximated by the bond valence sum as a parameter that is as close as possible to the integral value or “formal oxidation number“ [19, 20].
The valence of a bond is the measure of interaction between electrons sharing the bonding of atoms. For the amorphous materials, the coordination spheres were limited [21, 22]. Beyond the distance, to the central atom around 6 Å, the refined parameters of dependence bond valance vs. interatomic distance do not change [23, 24]. Introducing chemical considerations, the concept of coordination requires estimating if an atom of a coordination array is bonded to the central atom or not. If such an atom significantly influences the interatomic distances of the remaining atoms of the array, it is primary bonded to the central atom.
For the big family of copper complexes, the concept of coordination sphere plasticity [25] and equatorial-axial influence [26] reflect such influence via the central atom. Both effects are manifested in structural correlations of bond distances within the chromophores of transition element compounds. The structural correlations of coordination compounds with CuO6 and CuN6 chromophores approach the asymptotical values with the shortest equatorial bond length at around 1.9 Å [25]. Small changes in coordination polyhedra may cause significant changes in a hydrogen-bonding network [27]. In most cases, electronic distortions do not violate the valence sum rule [28, 29]. Importing dynamic features into molecular chemistry requires shifting from “static” to “dynamic” covalent bonds leading from molecular to constitutional dynamic chemistry [30].
As the magnetic properties of linearly arranged trinuclear hydroxy-bridged copper(II) complexes studied by the methods of density functional theory (DFT) showed, the angle Cu–O–Cu and out-of-plane displacement of the hydrogen atoms at the bridge are the key magnet factors that determine their magnetic behavior [31]. The magneto-structural study of binuclear copper(II) benzoates shows the dominance of antiferromagnetic spin interaction between metallic centers through bridging carboxylates over direct Cu···Cu interaction [32, 33]. These facts suggest the crucial structural influence of copper-ligand interactions within the coordination polyhedra of the central and terminal metallic centers. Here we report the crystal structure and magnetic properties of a novel compound consisting of trinuclear copper entities containing the alkaloid derived from papaverine.
Experimental Section
Preparation of Crystals
The light green product of the entitled compound was prepared by heating papaverine (0.01 mol) with Cu(2-HOC6H4COO)2·2H2O (0.01 mol) in hot water and methanol. The mixture was stirred, filtered, and then left at room temperature. The solid product of the reaction was collected and recrystallized from hot methanol to give air-stable pale blue-turquoise crystals. The analytical data found/calculated are 12.42/12.54% (Cu), 53.40/53.74% (C), 4.40/4.6% (H), and 1.93/1.84% (N).
Crystal Structure Solution and Refinement
Single crystal data collection was performed on a STOE diffractometer at 100 K. Crystal data and structure refinement details are summarized in Table 1. The structure of1 was solved by the program SHELXS [34]. The structure was drawn by OLEX2 [35]. The refinements were carried out by SHELXL [36]. OMe group and water molecules disordered in two positions were refined under the constraint of their occupancy sums to unity. Positions of all hydrogen atoms were optimized under the constraint to ride on their parent atoms, with C–H bond length of 0.93 Å and O–H bond length of 0.82 Å.
Magnetic Measurements
Magnetic measurements were performed by a SQUID magnetometer MPMS-XL7 from Quantum Design in the RSO mode of detection. The temperature dependence of magnetic moment was recorded at 0.1 T as an external magnetic field and the temperature sweeping rate was set to 1 K/min. The magnetic susceptibility of the polycrystalline sample was measured by the Faraday method in the temperature range of 77–300 K. The mercury tetracyanato cobalt(II) was used as a calibrant [37].
Calculation Details
Bond Valence Calculations
….For idealized copper hexacoordination of four equidistant equatorial in-plane Cu–N, O3 bonds and two equidistant out-of-plane Cu–O′2 bonds (chromophore CuNO3O′2) the quantities rL, rS were defined. rS is the length of in-plane (equatorial) bonds and rL is the length of out-of-plane (axial) bonds. The bond valence sum (BVS) distribution was calculated as the sum of equatorial and axial contributions:
where sCu–O and sCu–N are the bond valence functions for Cu–O and Cu–N bonds [38]:
If BVS is kept constant, the contour of the function expressed by Eq. (1) with inserted Eq. (2) gives the dependence of rL vs. rS. For the idealized copper coordination of the chromophore Cun+NO3O′2 this dependence is
where parameter n is the formal charge of a copper cation, Ro,Cu(n)–O is the bond valence parameter of a copper-oxygen bond, and sCu–N, sCu(n)–O are the functions [39]:
The empirical bond-valence parameters Ro and b were used from the latest version of http://www.iucr.org/resources/data/datasets/bond-valence-parameters. Equation (4) is appropriate for ionic, covalent, and intermediate ionic-covalent bonding, respectively [40]. The predicted axial Cu–O′(2×) bond length of the copper coordination of Cu2+O4O′2 chromophore was calculated as a numerical solution of the Eqs. [41, 42]:
where BVSeq is the bond valance sum of equatorial Cu–O bonds calculated by Eq. (2). αi are the parameters of the function (2) for Cu–O bonds.
Magnetic Measurements
The correction for the diamagnetism of the constituent atoms was calculated by Pascal’s constants [43]. The effective magnetic moments were calculated using the equation:
To assess the values of magnetic exchange parameters the broken-symmetry DFT approach was employed. Major –O–CH3 (OMe) groups were used in calculations. The magnetic parameters were used in the form −JijŜiŜj. The final values of magnetic parameters were extracted using the adapted Eq. (7) of Yamaguchi and co-workers, for broken symmetry (BS) and high spin (HS) states [44, 46]:
The fitting of the magnetic susceptibility and magnetization in 1 was performed with the program.
PHI 3.1.1 [47]. Before the quantum-chemistry calculations, the positions of all hydrogen atoms were optimized using the method PBEh-3c [48]. All other atoms were kept in their positions as obtained from the crystal structure investigation. Calculation of magnetic exchange parameters was carried out within the program ORCA 4.2.0 [49, 50]. The second-order Douglass–Kroll–Hess correction (DKH2) [51] and the chain-of-spheres approximation (RIJCOSX) [52] were set on. For all atoms, accounting for scalar relativistic effects Ahlrichs’s basis set DKH-def2-TZVP [53] was used with an auxiliary basis set def2/J [54]. The magnetic exchange parameters were obtained from DFT calculation utilizing the hybrid functional B3LYP [55,56,57,58]. In all calculations, the increased integration and fitting grids were used (grid6 and gridx6 in ORCA convention).
Results and Discussion
Crystal Structure
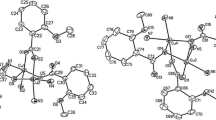
The crystal structure of compound 1 is composed of the tri copper units [Cu3(C7H5O3)4(C20H20NO5)2(H2O)2] (2) presented in Fig. 1 and noncoordinated water molecules.
The structure of complex 2 with the numbering of nonhydrogen atoms. The displacement ellipsoids are drawn at the 50% probability level. The equatorial mean planes of copper coordination polyhedra are shaded. The relative configuration at the C16 centers [59] is mentioned
Selected bond lengths and angles are collected in Table 2. N, O -bonded(6,7-dimethoxyisoquinolin-1-yl)-(3,4-dimethoxyphenyl)-methanolato ligand in this work is named papaverinyloxy ligand. The trinuclear unit shows chirality at C16. One OMe group attached to the phenyl ring is disordered in two positions of an occupancies ratio of 0.777(3)/0.223(3). The second OMe group is partially stabilized by the hydrogen bond.
OW2–HW4‧‧‧O10, while its relative free methyl group is split in two positions of an occupancies ratio 0.500(3) /0.500(3). The noncoordinated water molecules of oxygens OW2, and OW3 are disordered in two crystal sites with an occupancy ratio of 0.730(6) /0.270(6). The isoquinoline and phenyl rings of the papaverinyloxy ligand are significantly deviated from planarity. The angle between the least-squares planes of isoquinoline ring and the phenyl ring is 87.19(7)°. In papaverine hydrochloride, the corresponding angle is 80.13(8)°.
In the structure of salicylic acid [60] the corresponding deviation is 0.0012(1) Å. The phenyl rings of 2-hydroxybenzoates do not deviate from planarity. But the atoms O6 of hydroxyl groups deviate from the least-squares planes of phenyl rings by 0.054(2) Å. The complex units 2 are connected via hydrogen bonds of OW2 water molecules connected into the infinite chains along [101] direction (Fig. 2). The phenyl rings of adjacent symmetry-related 2-hydroxybenzoates assemble the complex units via π···π stack interactions [61, 62] into the chains along the [010] direction (Fig. 3). The hydrogen bond lengths are presented in Table 3.
The inter-centroid distance and parallel shifts of phenyl rings are 3.7379(1) Å and 1.66901(5) Å. Slightly distorted trans-square coordination of Cu1 is created by two in-plane carboxylic O atoms and two oxygens of papaverinyloxy ligands. The five-membered ring containing Cu2 is slightly deviated from the planar geometry. The torsion angle N1–C1–C16–O7 is 18.3(3)°. The Cu2–O3 bond stabilizes the copper coordination of the 2-hydroxybenzoato ligand while the atom O2 remains free.
The coordination polyhedron around Cu2 can be described as a distorted tetragonal bipyramid. The equatorial bonds of its inner coordination are Cu2–N1i, O1, O4, and O7i. The greatest deviation from the least-squares plane defined by the donor atoms is shown by atom N1i [0.079(2) Å]. Atom Cu2 is displaced from the basal plane toward the atom OW1 by 1.1406(1) Å. The hexacoordination of atom Cu2 is completed by the axial bonds Cu2–OW1 and Cu2–O3 of 2.445(2) Å and 2.872(2) Å, respectively. The angle formed by the Cu2–O3 bond and the basal least-squares plane of the coordination polyhedron around Cu2 is 83.56(5)°.
Bond Valence Analysis
For copper atom valence 2+, the predicted lengths of Cu1–OW1 bonds calculated by Eq. (5) is 2.50(2) Å which does not deviate significantly from the experimental bond length of 2.497(2) Å. The coordination of Cu1 is completed by two out-of-plane water O atoms to an axially distorted tetragonal bipyramid of chromophore CuO4O′2. The bond valence sum of Cu1 calculated by formulae (2) is 2.05(2) v. u. Figure 4 shows the contour graph of the copper atom valence (BVS) for idealized copper coordination of CuNO3O′2 chromophore. The numeric labels of isolines are the BVS values which are kept constant for relevant rL vs. rS dependence. The maximum bond lengths are rS(max) = 2.92(1) Å and rL(max) = 3.07(3) Å.
Diagram RLvs. RS (in Å) for the copper complexes of chromophore CuNO3O′2 compared with the calculated rLvs. rS dependences. Solid lines - contour graph of the function expressed by the Eqs. (1) and (2). Dashed lines – BVS functions expressed by the Eq. (3). ○ – model structures retrieved in CSD. ▲- the structure of 1. Spearman rank-order correlation coefficient [63] is − 0.844
For those distances, the contributions of equatorial and axial bonds to BVS in Eq. (1) yield zero values. In the regions of distances, the rS > rS(max) and rL > rL(max) these contributions are negative. The bond lengths rS(max) and rL(max) are the supreme limits of bonding distances within the inner copper coordination. If the bond valence sum of a copper atom is kept constant, any increase in the distance rS causes a decrease of rL and vice versa, i.e. such distortion of the inner coordination is plastic. 293 independent rS, rL values of the structures retrieved in CSD [64, 65] were approximated by the means of equatorial bond lengths (RS) and axial bond lengths (RL) [66]. The model structures were identified by the connectivity shown in Scheme 2. The standard atomic radii and tolerances of CSD were used. Only the non-disordered structures with an R-factor less than 0.05 were accepted. The structures were inspected by the program ORTEP-3 for Windows [67]. REFCODES of model structures with RS and RL values are included in the Supplementary Information. The best approach to the contour line and the graph of Eq. (3) for the copper oxidation state 2.0 show points in the region rL ≥ rS. The weak bonds Cu2–O3 are included in the inner coordination of Cu2. The bond valences of these bonds calculated by Eq. (2) are 0.14(1) and 0.030(7) v.u. The bond valence sum of Cu2 is 2.08(1) v. u.
Magnetic Properties
In the trinuclear complex unit, three magnetic exchange pathways are to be considered: Pathways between the metallic centers Cu2‧‧‧Cu1, Cu1‧‧‧Cu2i and the pathway between more distant terminal copper atoms Cu2‧‧‧Cu2i (Fig. 1). The resulting parameters of magnetic interaction are J12 = J23 = + 75.8 cm−1, and J13 = + 0.4 cm−1. The resulting Mullican spin densities are collected in Table 4.
As apparent, the DFT calculation predicts strong ferromagnetic interaction between adjacent centers in contrast with negligible interaction between the terminal copper centers. The resulting values were used as input for the following analysis of experimental magnetic functions of 1. The temperature and field dependence of magnetic functions are presented in Fig. 5.
The increase of susceptibility product at low temperatures indicates the presence of ferromagnetic exchange interaction, while the sudden decrease below 10 K can be ascribed to the intermolecular antiferromagnetic interaction (molecular field). The magnetic functions were analyzed according to the following spin Hamiltonian [68]:
where mB is the Bohr magneton, gi is the average value of the individual g-factors, Ŝiz is the projection of the spin operator of the i-th center, and zJ is the parameter of the molecular field. The two parameters of magnetic exchange interaction were fixed equal and the parameter between the terminal copper center Cu2 and Cu21 was completely omitted based on the DFT assessment (vide supra). The constraint g1 = g3 is due to the symmetry of complex 2. As can be seen, the fitted and calculated values of magnetic exchange interaction are in very good agreement. The g-factors of the magnetic centers gain typical values for copper(II) centers.
Conclusions
The trinuclear 2-hydroxybenzoato copper(II) complex with (6,7-dimethoxy-isoquinolin-1-yl)-(3,4-dimethoxy-phenyl)-methanol has been prepared and characterized. The copper atoms of the centrosymmetric trinuclear complex 2 are hexacoordinated. The novel N, O -bonded(6,7-dimethoxyisoquinolin-1-yl)-(3,4-dimethoxyphenyl)-methanolato (papaverinyloxy) ligands are bidentate. One-half of 2-hydroxybenzoates connect atoms Cu1 and Cu2 via bridging –COO groups. The remaining 2-hydroxybenzoates are bonded to the atoms Cu2 by the oxygens of nonbridging carboxylate groups. The positions of their phenyl rings are stabilized by the weak bonding of Cu2 atoms with the hydroxyl groups. A significant difference in Cu1 and Cu2 coordination geometries is consistent with the plasticity of the inner copper coordination of CuO4O’2 and CuNO3O′2 chromophores. The supreme limit for the equidistant equatorial bonds of the last chromophore is 2.92(1) Å. For the equidistant axial bonds, the supreme limit is 3.07(3) Å. If RL≥RS, the dependence RL vs. RS of copper inner coordination of Cun+NO3O′2 chromophore can be approximated by Eq. (3). The magnetism of complex 2 can be well explained by strong ferromagnetic interaction between the neighboring magnetic centers via the dominant pathways Cu1–O5–C35–O4–Cu2, Cu1–O7–Cu2i, and Cu1–OW1–Cu2. The plasticity of inner copper coordination and the papaverinyloxy ligand facilitate the formation of the trinuclear copper complex 2. By the copper activation of a papaverine methyl C–H bond, the hydrogen atom is replaced with bridging oxygen.
Supplementary Information
X-ray crystallographic data for the title compound (CCDC No.1999284) have been deposited with the Cambridge Crystallographic Data Center (http://www. ccdc.cam.ac.uk/).The datasets generated during and/or the current study are available from the corresponding author upon reasonable request.
Data Availability
The authors declare thet all other data supportig the findings of this study are available within the article and its supplementary information files.
References
Slavík J, Slavíková L (1994) Alkaloids from Papaver pinnatifidum Moris. Collect Czech Chem Commun 59:1879–1883
Utkan T, Sarioglu Y, Utkan NZ, Gönüllü NN, Yildirim MK (1996) Vascular smooth muscle reactivity and endothelium derived relaxing factor in experimental obstructive jaundice. Arch Physiol Biochem 104:30–35. https://doi.org/10.1076/apab.104.1.30.12864
Pavel IZ, Heller L, Sommerwerk S, Loesche A, Al-Harrasi A, Csuk R (2016) Drotaverine a concealed cytostatic! Arch Pharm 350:e1600289. https://doi.org/10.1002/ardp.201600289
Czyrski A, Girreser U, Hermann T (2013) 15N NMR chemical shifts in papaverine decomposition products. J Mol Struct 1036:111–114. https://doi.org/10.1016/j.molstruc.2012.09.072
Girreser U, Hermann TW, Piotrowska K (2003) Oxidation and degradation products of papaverine, part II[1]: investigations on the photochemical degradation of papaverine solutions. Arch Pharm Med Chem 336:401–405. https://doi.org/10.1002/ardp.200300751
Piotrowska K, Hermann TW, Augustyniak W (2006) Kinetics of photooxidation of papaverine hydrochloride and its major photooxidation products. J Pharm Biomed Anal 41:1391–1395. https://doi.org/10.1016/j.jpba.2006.02.036
Hermann TW, Girreser U, Michalski P, Piotrowska K (2002) Oxidation and degradation products of papaverine. Part I: Gadamer and Schulemann’s papaverinol synthesis revisited. Archive Der Pharmazie 4:167–169.
Reynolds CD, Palmer RA, Gorinsky B (1973) Crystal and molecular structure of the alkaloid papaverine hydrochloride. J Cryst Mol Struct 4:213–218
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Advances in copper complexes as anticancer agents. Am Chem Soc Chem Rev. https://doi.org/10.1021/cr400135x
Zeleňák V, Sabo M, Massa W, Černák J (2004) Tetra-µ-benzoato-κ 8O:O′-bis(1-[(3,4-dimethoxyphenyl)methyl]-6, 7-dimethoxyisoquinoline-κNzinc(II)): the first crystal structure with papaverine as a ligand. Acta Crystallogr Sect C: Cryst Struct Commun 60:m85–m87. https://doi.org/10.1107/S010827010302883X
Catterick J, Thornton P (1977) Structures and physical properties of polynuclear carboxylates. Adv Inorg Chem Radiochem 20(08):291–362. https://doi.org/10.1016/S0065-2792
Melník M (1981) Mono-, bi-, tetra- and polynuclear copper(II) halogenocarboxylates. Coord Chem Rev 36:1–44. https://doi.org/10.1016/S0010-8545(00)80504-4
Melník M (1981) Binuclear caffeine adducts of Cu(II) acetate and cu(II) chloroacetates with unusually high antiferromagnetic interaction. J Inorg Nucl Chem 43:3035–3038. https://doi.org/10.1016/0022-1902(81)80677-X41
Melník M (1982) Structural isomerism of copper(II) compounds. Coord Chem Rev 47:239–261. https://doi.org/10.1016/0010-8545(82)85033-9
Alig H, Trömel M (1992) Geometrische und chemische koordination. Z für Kristallographie 201:213–222. https://doi.org/10.1524/zkri.1992.201.3-4.213
Werner A (1893) Beitrag Zur Konstitution Anorganischer Verbindungen. Z für Anorganische Chemie 3:267–330. https://doi.org/10.1002/zaac.18930030136
Gopinathan MS, Jug K (1983) Valency. I. A quantum chemical definition and properties. Theoret Chim Acta (Berl) 63:497–509. https://doi.org/10.1007/BF02394809
Gopinathan MS, Jug K (1983) Valency. II. Applications to molecules with first-row atoms. Theoret Chim Acta (Berl) 63:511–527. https://doi.org/10.1007/PL00020125
Jørgensen CK (1969) Stereochemistry and the choice between alternative descriptions of oxidation states. Chem Phys Lett 5:150–151. https://doi.org/10.1016/0009-2614(69)80142-9
Jørgensen CK (1969) Oxidation numbers and oxidation states. Springer, Berlin, Heidelberg, New York
Adams S (2001) Relationship between bond valence and bond softness of alkali halides and chalcogenides. Acta Cryst B 57:278–287. https://doi.org/10.1107/S0108768101003068
Chen H, Adams S (2017) Bond softness sensitive bond-valence parameters for crystal structure plausibility tests. IUCrJ 4:614–625. https://doi.org/10.1107/S2052252517010211
Brown ID (2017) What is the best way to determine bond-valence parameters? IUCrJ 4:514–515. https://doi.org/10.1107/s2052252517011782
Alcock NW (1972) Secondary bonding to nonmetallic elements. Advan Inorg Chem Radiochem 15(08):1–58. https://doi.org/10.1016/S0065-2792
Gažo J, Bersuker IB, Garaj J, .Kabešová M, Kohout J, Langfelderová M, Melník M, Serátor M, Valach F (1976) Plasticity of the coordination sphere of copper(II) complexes, its manifestation and causes. Coord Chem Rev 19:253–298. https://doi.org/10.1016/S0010-8545(00)80317-3
Gažo J, Boča R, Jóna E, Kabešová M, Macášková L, Šima J, Pelikán P, Valach F (1982) Equatorial-axial interactions in complexes as a manifestation of mutual influence of ligands. Coord Chem Rev 43:87–131. https://doi.org/10.1016/S0010-8545(00)82093-7
Sundberg MR (2000) Effect of hydrogen bonding on coordinate bond. Semi-coordination in Werner-type copper(II) complexes revisited. Rev Inorg Chem 20:195–218. https://doi.org/10.1515/REVIC.2000.20.3.195
Brown ID (1992) Chemical and steric constraints in inorganic solids. Acta Cryst 48:553–572. https://doi.org/10.1107/S0108768192002453
Brown ID (1981) The Bond-Valence Method: An Empirical Approach to Chemical Structure and Bonding. In: Structure and Bonding in Crystals. Edited by M. O’Keefee & A. Navrotsky, New York: Acadamic Press, Inc. pp. 1–30
Lehn JM (2012) Constitutional dynamic chemistry: Bridge from supramolecular chemistry to adaptive chemistry. Top Curr Chem 322:1–32. https://doi.org/10.1007/128_2011_256
Rodríguez-Fortea A, Ruiz E, Alemany P, Alvarez S (2003) Magneto-Structural correlations in trinuclear Cu(II) complexes: a density functional study. Monatshefte Chem 134:307–316. https://doi.org/10.1007/s00706-002-0523-6
Kawata T, Uekusa H, Ohba S, Furukawa T, Tokii T, Muto Y, Kato M (1992) Magneto-structural correlation in dimeric copper(II) benzoates. Acta Cryst B48:253–261. https://doi.org/10.1107/S0108768191012697
Yamanaka M, Uekusa H, Ohba S, Saito Y, Iwata S, Kato M, Tokii T, Muto Y, Steward OW (1991) Correlation of electron density and spin-exchange interaction in dimeric copper(II) formates, acetates and silanecarboxylates. Acta Cryst B 47:344–355. https://doi.org/10.1107/S0108768190012459
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122. https://doi.org/10.1107/S0108767307043930
Dolomanov OV, Bourhis RJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341. https://doi.org/10.1107/S0021889808042726
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Figgis BN, Nyholm RS (1958) A convenient solid for calibration of the Gouy magnetic susceptibility apparatus. J Chem Soc 4:190–4191
Valach F (1999) A bond-valence approach to the semicoordination of copper-oxygen and copper-nitrogen complexes. Polyhedron 18:699–706. https://doi.org/10.1016/S0277-5387(98)00342-8
Brown ID, Altermatt D (1985) Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal structure database. Acta Cryst B41:244–247. https://doi.org/10.1107/S0108768185002063
Urusov VS (1995) Semi-empirical groundwork of the bond‐valence model. Acta Cryst B51:641–649. https://doi.org/10.1107/S0108768195003417
Valach F, Tokarčík M, Maris T, Watkin DJ, Prout CK (2000) Bond-valence approach to the copper-copper and copper-oxygen bonding in binuclear copper(II) complexes: structure of tetrakis(2-fluorobenzoato-O,O′)-bis(2-fluorobenzoate-O) dicopper(II). Z Kristallogr 215:56–60. https://doi.org/10.1524/zkri.2000.215.1.56
Valach F, Tokarčík M, Saunders A, Cowley A, Watkin DJ (2001) Structural isomerism of propionato copper(II) complex: Crystal and molecular structure of tetrakis(µ-o-propionato)bis(methyl 3-pyridyl-N-carbamate)dicopper(II) at 190 K. Polyhedron 20:1933–1937. https://doi.org/10.1016/S0277-5387(01)00784-7
König E (1966) Magnetic properties of coordination and organometallic transition metal compounds. Springer-, Berlin
Yamaguchi K, Takahara Y, Fueno T (1986) In: Applied Quantum Chemistry, V. H. Smith, V. Reidel (Ed.), Dordrecht, p. 155
Soda T, KitagawaY, Onishi T, Takano Y, Shigeta Y, Nagao H, Yoshika Y, Yamaguchi K (2000) Ab initio computations of effective exchange integrals for H-H, H-He-H and Mn2O2 complex: comparison of broken-symmetry approaches. Chem Phys Lett 319:223–230. https://doi.org/10.1016/S0009-2614(00)00166-4
Neese F (2009) Prediction of molecular properties and molecular spectroscopy with density functional theory: from fundamental theory to exchange-coupling. Coord Chem Rev 253:526–563. https://doi.org/10.1016/j.ccr.2008.05.014
Chilton NF, Anderson RP, Turner LD, Soncini A, Murray KS (2013) PHI: a powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J Comput Chem 34:1164–1175. https://doi.org/10.1002/jcc.23234
Grimme S, Brandenburg JG, Bannwarth C, Hansen A (2015) Consistent structures and interactions by density functional theory with small atomic orbital basis sets. J Chem Phys 143:54107–54119. https://doi.org/10.1063/1.4927476
Neese F (2012) The ORCA program system. WIREs Comput Mol Sci. https://doi.org/10.1002/wcms.81
Neese F (2018) Software update: the ORCA program system, version 4.0. WIREs Comput Mol Sci. https://doi.org/10.1002/wcms.1327
Nakajima T, Hirao K (2012) The Douglas-Kroll-Hess approach. Chem Rev 112:385–402. https://doi.org/10.1021/cr200040s
Izsák R, Neese F (2011) An overlap fitted chain of spheres exchange method. J Chem Phys 135:144105–114411. https://doi.org/10.1063/1.3646921
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/b508541a
Weigend F (2006) Accurate coulomb-fitting basis sets for H to Rn. Phys Chem Chem Phys 8:1057–1065. https://doi.org/10.1039/b515623h
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464304
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200–1211
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Cahn RS, Ingold Ch Prelog V (1966) Specification of molecular chirality. Angew Chem Intern Edite 5:385–415. https://doi.org/10.1002/anie.196603851
Munshi P, Guru Row TN (2006) Intra- and intermolecular interactions in small bioactive molecules: Cooperative features from experimental and theoretical charge-density analysis. Acta Cryst B62:612–626
Janiak C (2000) A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J Chem Soc Dalton Trans. https://doi.org/10.1039/b003010o
Mooibroek TJ, Gamez P (2007) The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg Chim Acta 360:381–404. https://doi.org/10.1016/j.ica.2006.07.061
Havlicek LL, Crain RD (1988) Practical statistics for the Physical sciences. American Chemical Society, Washington DC, pp 109–110
Allen FH (2002) The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Cryst B. https://doi.org/10.1107/S0108768102003890
Allen FH, Motherwell WDS (2002) Applications of the Cambridge Structural Database in organic chemistry and crystal chemistry. Acta Cryst B 58:407–422. https://doi.org/10.1107/S0108768102004895
Tomlinson AAG, Hathaway BJ, Billing BE, Nichols P (1969) The electronic properties and stereochemistry of the copper(II) ion. Part V. The tetra-ammine complexes. J Chem Soc A. https://doi.org/10.1039/J19690000065
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Cryst 45:849–854. https://doi.org/10.1107/S0021889812029111
Boča R (1999) Theoretical foundations of Molecular Magnetism. Elsevier Science & Technology Oxford
Acknowledgements
The article was created with the support of the Ministry of Education, Science, Research and Sport of the Slovak Republic within the Research and Development Operational Programme for the project “University Science Park of STU Bratislava”, ITMS 26240220084, co-funded by the European Regional Development Fund. We are also grateful to the HPC center at the Slovak University of Technology in Bratislava, which is a part of the Slovak Infrastructure of High-Performance Computing [SIVVP project, ITMS code 26230120002, funded by the European regional development funds (ERDF)]. We are grateful to the HPC center at the Slovak University of Technology in Bratislava, which is a part of the Slovak Infrastructure of High Performance Computing (SIVVP project, ITMS code 26230120002, funded by the European region development funds, ERDF), for the computational time and resources made available. This article was created with the support of the Ministry of Education, Science, Research and Sport of the Slovak Republic within the Research and Development Operational Programme. for the project “University Science Park of STU Bratislava”, ITMS 26240220084, for funding within the scheme Excellent research teams, co-funded by the European Regional Development Fund. The authors are grateful to Professor Tibor Gracza, and Dr. Daniel Vegh, for preliminary discussions.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. Funding for this research was provided by: Vedecká Grantová Agentúra MŠVV M SR a SAV (VEGA) (Grant No. 1/0175/23; Grant No. 1/0029/22), Agentúra na Podporu Vedy a Výskumu (Grant No. 20–0213, Grant No. 19–0087, Grant No. 22-0172, Grant No. DS-FR-22-0010).
Author information
Authors and Affiliations
Contributions
FV solved and refined the crystal structure and construcrted the structure correlations, JP and IŠ interpreted the data of magnetical measurements and quantum mechanical calculations, MM prepared the single crystal, JK refined the final structure model, FV and JP wrote the manuscript together.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Valach, F., Pavlik, J., Šalitroš, I. et al. Novel Trinuclear Copper(II) Complex: Crystal Structure at 100 K and Magnetic Properties of (R, S)-di[(6,7-dimethoxy-isoquinolin-1-yl)-(3,4-dimethoxy-phenyl)-methanolato]-tetra(2-hydroxybenzoato)-diaqua-tricopper dihydrate, [Cu3(C7H5O3)4(C20H20NO5)2(H2O)2]·2(H2O). J Chem Crystallogr 54, 163–172 (2024). https://doi.org/10.1007/s10870-024-01009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-024-01009-2