Abstract
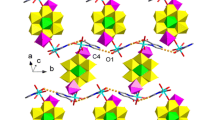
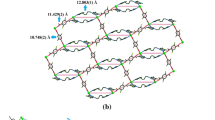
The preparation and characterization of two supramolecular complexes from octahedral [Ta6Cl12(CN)6]4−/3− units and 2, 2′: 6′, 2″-terpyridine (Terpy) metal complexes as building blocks are reported. Single crystal analyses revealed [Mn(Terpy)]2[Ta6Cl12(CN)6]·MeOH (1) features a neutral two-dimensional (2D) framework composed of layers based on [Ta6Cl12(CN)6]4− and [Mn(Terpy)]2+. Each layer is built of 6-connected [Ta6Cl12]2+ and 3-connected Mn(II) nodes bridged by cyanide ligands. The layers are held together by MeOH molecules. The layered structure is maintained after the removal of solvent molecule (MeOH) upon heating, leading to the formation of [Mn(Terpy]2[Ta6Cl12(CN)6] (1′). In the case of using Gd3+, the resultant product was revealed by single-crystal analyses to be an ionic compound [Gd(Terpy)(H2O)4(DMF)2][Ta6Cl12(CN)6]⋅DMF ⋅3H2O (2). 2 has a three-dimensional (3D) framework built of [Gd(Terpy)(H2O)4(DMF)2]3+ and [Ta6Cl12(CN)6]3− ions connected to each other via extensive hydrogen bonds and π-π interactions between the cations, anions, and solvent molecules. Thermal stabilities of 1‒2 are reported.
Graphical Abstract
The X-ray structures and thermal stabilities of a coordination polymer [Mn(Terpy)]2[Ta6Cl12(CN)6] ⋅MeOH and an ionic compound [Gd(Terpy)(H2O)4(DMF)2][Ta6Cl12(CN)6]⋅DMF⋅3H2O are presented.





Similar content being viewed by others
Data Availability
The online version of this article contains supplementary material that are available to authorized users.
References
Williams GT, Haynes CJE, Fares M, Caltagirone C, Hiscock JR, Gale PA (2021) Chem Soc Rev 50:2737–2763
Steed JW, Atwood JL (2022) Supramolecular Chemistry, 3rd edn. Wiley
Olivo G, Capocasa G, Giudice DD, Lanzalunga O, Stefano SD (2021) Chem Soc Rev 50:7681–7724
Wu D, Zhang PF, Yang GP, Hou L, Zhang WY, Han YF, Liu P, Wang YY (2021) Coord Chem Rev 434:213709
Hu ZG, Wang YX (2021) Zhao D 50:4629–4683
Dong XY, Si YB, Yang JS, Zhang C, Han Z, Luo P, Wang ZY, Zang SQ, Mak TCW (2020) Nature Comm 11:3678
Che CM, Lai SW (2005) Coord Chem Rev 249:1296–1309
Farley CM, Uyeda C (2019) Trends Chem 1:497–509
Fedorov VE, Naumov NG (2019) Ligated transition metal clusters in solid-state chemistry. Springer Nature Switzerland AG, Cham
Lemoine P, Halet JF, Cordier S (2019) Ligated transition metal clusters in solid-state chemistry. Springer Nature Switzerland AG, Cham
Litvinova YM, Gayfulin YM, Kovalenko KA, Samsonenko DG, van Leusen J, Korolkov IV, Fedin VP, Mironov YV (2018) Inorg Chem 57:2072–2084
Daigre G, Lemoine P, Pham TD, Demange V, Gautier R, Naumov NG, Ledneva A, Amela-Cortes M, Dumait N, Audebrand N, Cordier S (2018) CrystEngComm 20:3396–3408
Zhang JJ, Zhou HJ, Lachgar A (2007) Angew Chem Int Ed 46:4995–4998
Zhang JJ, Lachgar A (2015) Inorg Chem 54:1082–1090
Shamshurin MV, Mikhaylov MA, Sukhikh T, Benassi E, Tarkova AR, Prokhorikhin AA, Kretov EI, Shestopalov MA, Abramov PA, Sokolov MN (2019) Inorg Chem 58:9028–9035
Renaud A, Wilmet M, Truong TG, Seze M, Lemoine P, Dumait N, Chen W, Saito N, Ohsawa T, Uchikoshi T, Ohashi N, Cordier S, Grasset F (2017) J Mater Chem C 5:8160–8168
Przychodzen P, Pelka R, Lewinski K, Supel J, Rams M, Tomala K, Sieklucka B (2007) Inorg Chem 46:8924–8938
Elahi SM, Raizada M, Sahu PK, Konar S (2021) Chem Eur J 27:5858–5870
Bajan B, Balzer G, Meyer HJ (1997) Z Anorg Allg Chem 623:1723–1728
GADDS V4.1.14 General area detector diffraction system program for instrument control and data collection. BRUKER AXS Inc., Madison
EVA V8.0 Graphics program for 2-dimensional data evaluation and presentation. BRUKER AXS Inc., Madison
Shamshurin MV, Abramov PA, Mikhaylov MA, Sokolov MN (2022) J Struct Chem 63:81–86
Gulyaeva RI, Petrovab SA, Chumarev VM, Selivanov EN (2020) J Alloys Comps 834:155153
Khitrova VI, Klechkovskaya VV, Pinsker ZG (1967) Kristallografiya 12:1044
Rooksby HP, White EAD, Langston SA (1965) J Am Ceram Soc 48:447–449
Ustimovich AB, Pinaeva MM, Kuznetsova VV, Vasil’ev VS (1977) Inorg Mater 13:120
Zhou HJ, Lachgar A (2007) Eur I Inorg Chem 8:1053–1066
Acknowledgements
This work is supported by the National Science Foundation under Grant Nos. DMR-0446763 and 0234489. We appreciate Dr. Cynthia Day for her continuous help in crystallography/data collection and wish her a happy retirement.
Author information
Authors and Affiliations
Contributions
Jian-Jun Zhang wrote the original manuscript. H. Andrew Zhou and Abdessadek Lachgar revised the manuscript. H. Andrew Zhou finalized the manuscript and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, JJ., Zhou, H.A. & Lachgar, A. Supramolecular Complexes Built of Octahedral [Ta6Cl12(CN)6]3−/4− Clusters and Terpyridine-Metal Complexes. J Chem Crystallogr 53, 299–306 (2023). https://doi.org/10.1007/s10870-022-00969-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00969-7