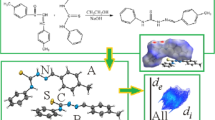
Abstract
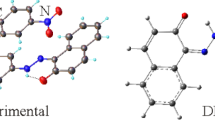
New hydrazone derivate, (1Z,2E)-2-(2-(1-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)ethyl)hydrazineylidene)-2-(p-tolyl)acetaldehyde oxime (H2L) was synthesized by 5-acetyl-1,3-dimethyl-barbituric acid and p-methyl isonitrosophenylhydrazine. Its molecular and crystal structures were determined by single crystal X-ray analysis. It belongs to triclinic system P-1 space group with a = 7.1722 (3) Å, b = 10.5362 (4) Å, c = 11.7675 (5) Å, α = 98.844 (4)°, β = 98.882 (4)°, γ = 104.330 (4)°, Z = 2 and V = 833.95 (6) Å3. In the molecular structure, the intramolecular N–H···O and N–H···N hydrogen bonds enclose S(6) ring motifs. In the crystal structure, the intermolecular C–H···O and O–H···O hydrogen bonds link the molecules into centrosymmetric dimers, enclosing R22(10) and R44(10) ring motifs, in which they may be effective in stabilization of the structure. The Hirshfeld surface analysis of crystal structure indicates that the most important contributions for crystal packing are from H…H (48.5%), H…O/O…H (23.7%) and H…C/C…H (9.7%) interactions. Hydrogen bonding and van der Waals interactions are the dominant interactions in crystal packing. Computational chemistry indicates that in the crystal, O–H···O and C–H···O hydrogen bond energies are 95.9 and 87.5 kJ mol−1. The evaluation of the electrostatic, dispersion and total energy frameworks indicates that stabilization is dominated via the nearly equal strengths of the electrostatic and dispersion energy contributions.
Graphical Abstract











Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Barelli A, Biondi I, Soave M, Tafani C, Bononi F (2008) The comprehensive medical preparedness in chemical emergencies: ‘the chain of chemical survival.’ Eur J Emerg Med 15(2):110–118. https://doi.org/10.1097/MEJ.0b013e3280bef902
Angelusiu MV, Barbuceanu SF, Draghici C, Almajan GL (2010) New Cu (II), Co (II), Ni (II) complexes with aroyl-hydrazone based ligand. Synthesis, spectroscopic characterization and in vitro antibacterial evaluation. Eur J Med Chem 45(5):2055–2062. https://doi.org/10.1016/j.ejmech.2010.01.033
Savini L, Chiasserini L, Gaeta A, Pellerano C (2002) Synthesis and anti-tubercular evaluation of 4-quinolylhydrazones. Bioor Med Chem 10(7):2193–2198. https://doi.org/10.1016/S0968-0896(02)00071-8
Giziroglu E, Sarikurkcu C, Sarac N (2015) Synthesis and characterization of novel Hydrazone based anti-mutagenic and antioxidative agents. J Appl Pharm Sci 5(3):048–055. https://doi.org/10.7324/JAPS.2015.510.S9
Chugunova E, Voloshina A, Mukhamatdinova R, Serkov I, Proshin A, Gibadullina E, Goumont R (2014) The study of the biological activity of amino-substituted benzofuroxans. Lett Drug Des Discov 11(4):502–512
Glinma B, Gbaguidi FA, Urbain CK, Kpovıessı SD, HOUNGBEME A, HOUNGUE H D, POUPAERT JH, (2015) Synthesis and trypanocidal activity of salicylhydrazones and p-tosylhydrazones of S-(+)-carvone and arylketones on African trypanosomiasis. J Appl Pharm Sci 5(06):001–007. https://doi.org/10.7324/JAPS.2015.50601
Mahmudov KT, Kopylovich MN, Maharramov AM, Kurbanova MM, Gurbanov AV, Pombeiro AJ (2014) Barbituric acids as a useful tool for the construction of coordination and supramolecular compounds. Coord Chem Rev 265:1–37. https://doi.org/10.1016/j.ccr.2014.01.002
Bojarski JT, Mokrosz JL, Bartoń HJ, Paluchowska MH (1985) Recent progress in barbituric acid chemistry. Adv Heterocycl Chem 38:229–297. https://doi.org/10.1016/S0065-2725(08)60921-6
Patrick GL (2013) An introduction to medicinal chemistry. Oxford University Press, Oxford
Kaminsky W, Jasinski JP, Woudenberg R, Goldberg KI, West DX (2002) Structural study of two N (4)-substituted thiosemicarbazones prepared from 1-phenyl-1, 2-propanedione-2-oxime and their binuclear nickel (II) complexes. J Mol Struct 608(2–3):135–141. https://doi.org/10.1016/S0022-2860(01)00921-8
Bertrand JA, Smith JH, Eller PG (1974) Crystal and molecular structure of bis perchlorato [2-(2-hydroxyethyl) imino-3-oximobutanato] aquocopper (II). Copper (II) dimer with bridging oxime groups. Inorg Chem 13(7):1649–1653. https://doi.org/10.1021/ic50137a021
Butcher RJ, O’Connor CJ, Sinn E (1979) Synthesis and relation between magnetism and structure of the binuclear copper (II) oxime complex [Cu2L2(ClO4)2].[Cu2L2(CH3OH)2](ClO4)2, where HL= 1-(N, N-dimethyl-2-aminoethylimino)-1-phenyl-2-oximopropane. Inorg Chem 18(7):1913–1918. https://doi.org/10.1021/ic50197a038
Abraham F, Capon JM, Nowogrocki G, Sueur S, Bremard C (1985) Synthesis, X-ray structure and spectroscopy of Cu (II) complexes derived from diacetylazine dioxime. Polyhedron 4(10):1761–1767. https://doi.org/10.1016/S0277-5387(00)84173-X
Maekawa M, Kitagawa S, Nakao Y, Sakamoto S, Yatani A, Mori W, Munakata M (1999) Syntheses, crystal structures and autoreduction behavior of antiferromagnetically coupled dicopper (II) oximato complexes. Inorg Chim Acta 293(1):20–29. https://doi.org/10.1016/S0020-1693(99)00215-7
Wan SP, Mori W, Yamada S (1986) Synthesis and properties of iodinated nickel imineoximes. Inorg Met-Org Chem 16(9):1273–1288. https://doi.org/10.1080/00945718608071400
Sutradhar M, Alegria EC, Mahmudov KT, da Silva MFCG, Pombeiro AJ (2016) Iron (III) and cobalt (III) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: catalysts for the microwave assisted oxidation of alcohols. RSC Adv 6(10):8079–8088. https://doi.org/10.1039/C5RA25774C
Sutradhar M, Martins LM, da Silva MFCG, Alegria EC, Liu CM, Pombeiro AJ (2014) Dinuclear Mn (II, II) complexes: magnetic properties and microwave assisted oxidation of alcohols. Dalton Trans 43(10):3966–3977. https://doi.org/10.1039/C3DT52774C
Sutradhar M, Martins LM, da Silva MFCG, Pombeiro AJ (2015) Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl Catal A 493:50–57. https://doi.org/10.1016/j.apcata.2015.01.005
Sutradhar M, Kirillova MV, da Silva MFCG, Liu CM, Pombeiro AJ (2013) Tautomeric effect of hydrazone Schiff bases in tetranuclear Cu (II) complexes: magnetism and catalytic activity towards mild hydrocarboxylation of alkanes. Dalton Trans 42(47):16578–16587. https://doi.org/10.1039/C3DT52453A
Sutradhar M, Martins LM, Guedes da Silva MFC, Mahmudov KT, Liu CM, Pombeiro AJ (2015) Trinuclear CuII structural isomers: coordination, magnetism, electrochemistry and catalytic activity towards the oxidation of alkanes. Eur J Inorg Chem 23:3959–3969. https://doi.org/10.1002/ejic.201500440
Sutradhar M, Alegria EC, Guedes da Silva MFC, Martins LM, Pombeiro AJ (2016) Aroylhydrazone Cu (II) complexes in keto form: structural characterization and catalytic activity towards cyclohexane oxidation. Molecules 21(4):425. https://doi.org/10.3390/molecules21040425
Mathews NA, Jose A, Kurup MP (2019) Synthesis and characterization of a new aroylhydrazone ligand and its cobalt (III) complexes: X-ray crystallography and in vitro evaluation of antibacterial and antifungal activities. J Mol Struct 1178:544–553. https://doi.org/10.1016/j.molstruc.2018.10.061
Banerjee S, Mondal S, Chakraborty W, Sen S, Gachhui R, Butcher RJ, Mitra S (2009) Syntheses, X-ray crystal structures, DNA binding, oxidative cleavage activities and antimicrobial studies of two Cu (II) hydrazone complexes. Polyhedron 28(13):2785–2793. https://doi.org/10.1016/j.poly.2009.05.071
Ma L, Li S, Zheng H, Chen J, Lin L, Ye X, Chen L (2011) Synthesis and biological activity of novel barbituric and thiobarbituric acid derivatives against non-alcoholic fatty liver disease. Eur J Med Chem 46(6):2003–2010. https://doi.org/10.1016/j.ejmech.2011.02.033
Laxmi SV, Janardhan B, Rajitha B, Raghavaiah P, Srinivas P (2012) Synthesis, single crystal X-ray studies and antimicrobial activities of novel Indole barbiturates. Med Chem Res 21(10):2896–2901. https://doi.org/10.1007/s00044-011-9827-6
Singh P, Kaur M, Verma P (2009) Design, synthesis and anticancer activities of hybrids of indole and barbituric acids—identification of highly promising leads. Bioorg Med Chem Lett 19(11):3054–3058. https://doi.org/10.1016/j.bmcl.2009.04.014
Shiradkar MR, Ghodake M, Bothara KG, Bhandari SV, Nikalje A, Akula KC, Burange PJ (2007) Synthesis and anticonvulsant activity of clubbed thiazolidinone–barbituric acid and thiazolidinone–triazole derivatives. ARKIVOC 14:58–74. https://doi.org/10.3998/ark.5550190.0008.e08
Goodman LS, Gilman A (1975) The pharmacological basis of therapeutics. Macmillan, New York
Segovia C, Lebrêne A, Levacher V, Oudeyer S, Brière JF (2019) Enantioselective catalytic transformations of barbituric acid derivatives. Catalysts 9(2):131
Parvathy KS, Negi PS, Srinivas P (2010) Curcumin–amino acid conjugates: synthesis, antioxidant and antimutagenic attributes. Food Chem 120(2):523–530. https://doi.org/10.1016/j.foodchem.2009.10.047
Abele E, Abele R, Golomba L, Višņevska J, Beresneva T, Rubina K, Lukevics E (2010) Oximes of six-membered heterocyclic compounds with two and three heteroatoms. II.* Reactions and biological activity. Chem Heterocycl Compd 46(8):905–930
Neumann DM, Cammarata A, Backes G, Palmer GE, Jursic BS (2014) Synthesis and antifungal activity of substituted 2, 4, 6-pyrimidinetrione carbaldehyde hydrazones. Bioorg Med Chem 22(2):813–826. https://doi.org/10.1016/j.bmc.2013.12.010
Zheng LW, Li Y, Ge D, Zhao BX, Liu YR, Lv HS, Miao JY (2010) Synthesis of novel oxime-containing pyrazole derivatives and discovery of regulators for apoptosis and autophagy in A549 lung cancer cells. Bioorg Med Chem Lett 20(16):4766–4770. https://doi.org/10.1016/j.bmcl.2010.06.121
Dörwald FZ (2012) Lead optimization for medicinal chemists: pharmacokinetic properties of functional groups and organic compounds. Wiley, Hoboken
Khan KM, Khan M, Ali M, Taha M, Hameed A, Ali S, Choudhary MI (2011) Synthesis and DPPH radical scavenging activity of 5-arylidene-N, N-dimethylbarbiturates. Med Chem 7(3):231–236. https://doi.org/10.2174/157340611795564231
Uysal Ş, Coşkun A, Koc ZE, Ucan M, Ucan HI (2007) Synthesis and characterization of some vic-dioxime and its mononuclear complexes. Russ J Coord Chem 33(5):351–357. https://doi.org/10.1134/S1070328407050077
Gup R, Giziroğlu E (2006) Metal complexes and solvent extraction properties of isonitrosoacetophenone 2-aminobenzoylhydrazone. Spectrochim Acta Part A 65(3–4):719–726. https://doi.org/10.1016/j.saa.2006.01.004
Koçak N, Sahin M, Ucan HI (2012) The synthesis of two new isonitrosoacetophenone derivatives and investigation of their Ni (II), Co (II), Cu (II), and Zr (IV) complexes. Russ J Inorg Chem 57(9):1227–1231. https://doi.org/10.1134/S0036023612090124
Jursic BS, Neumann DM (2001) Preparation of 5-formyl-and 5-acetylbarbituric acids, including the corresponding Schiff bases and phenylhydrazones. Tetrahedron Lett 42(48):8435–8439. https://doi.org/10.1016/S0040-4039(01)01830-5
Sheldrick GM (2015) SHELXT—integrated space-group and crystal-structure determination with. Acta Crystallogr Sect A Found Crystallogr A 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL with. Acta Crystallogr Sect C Found Crystallogr C 71:3–8. https://doi.org/10.1107/S2053229614024218
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Crystallogr 45(4):849–854. https://doi.org/10.1107/S0021889812029111
Spek AL (2009) PLATON/SQUEEZE. Acta Crystallogr Sect D Biol Crystallogr 65:148–155. https://doi.org/10.1107/S090744490804362X
Giziroglu E, Nesrullajev A, Orhan N (2014) 1, 3-Dimethyl-5-(3, 4, 5-tris (alkoxy) benzoyl) barbituric acid derivatives and their liquid crystalline difluoroboron complexes: Synthesis, characterization and comparative investigations of mesomorphic, thermotropic and thermo-morphologic properties. J Mol Struct 1056:246–253. https://doi.org/10.1016/j.molstruc.2013.10.038
Çelik TA, Sarikavakli N, Aslantürk ÖS (2019) In vitro cytotoxic and apoptotic effect of vic-dioxime ligand and its metal complexes. Appl Organometal Chem 33(4):e4818. https://doi.org/10.1002/aoc.4818
Jursic BS, Neumann DM, Bowdy KL, Stevens ED (2004) Simple, efficient, high yield syntheses of substituted and unsubstituted 5-benzoylbarbituric acids, and their corresponding schiff base phenylhydrazones. J Heterocycl Chem 41(2):233–246. https://doi.org/10.1002/jhet.5570410214
Giziroglu E, Aygün M, Sarikurkcu C, Kazar D, Orhan N, Firinci E, Gokcen C (2013) Synthesis, characterization and antioxidant activity of new dibasic tridentate ligands: X-ray crystal structures of DMSO adducts of 1,3-dimethyl-5-acetyl-barbituric acid o-hydroxybenzoyl hydrazone copper (II) complex. Inorg Chem Commun 36:199–205. https://doi.org/10.1016/j.inoche.2013.09.013
Etter MC, MacDonald JC, Bernstein J (1990) Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr B Struct Sci 46(2):256–262. https://doi.org/10.1107/S0108768189012929
Hirshfeld FL (1977) Synthesis, crystal structure, and Hirshfeld surface analysis of a new mixed ligand copper (II) complex. Theor Chim Acta 44(2):129–138. https://doi.org/10.1007/BF00549096
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. Cryst Eng Comm 11(1):19–32. https://doi.org/10.1039/B818330A
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17. University of Western Australia, Crawley
Venkatesan P, Thamotharan S, Ilangovan A, Liang H, Sundius T (2016) Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl) amino] prop-2-enoic acid. Spectrochim Acta Part A 153:625–636. https://doi.org/10.1016/j.saa.2015.09.002
Spackman MA, McKinnon JJ, Jayatilaka D (2008) Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 10:377–388. https://doi.org/10.1039/B715227B
Jayatilaka D, Grimwood DJ, Lee A, Lemay A, Russel AJ, Taylor C, Wolff SK, Cassam-Chenai P, Whitton A (2005) TONTO—a system for computational chemistry. Available at http://hirshfeldsurface.net/
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun 37:3814–3816. https://doi.org/10.1039/B704980C
Hathwar VR, Sist M, Jørgensen MR, Mamakhel AH, Wang X, Hoffmann CM, Iversen BB (2015) Quantitative analysis of intermolecular interactions in orthorhombic rubrene. IUCrJ 2(5):563–574. https://doi.org/10.1107/S2052252515012130
Turner MJ, Grabowsky S, Jayatilaka D, Spackman MA (2014) Accurate and efficient model energies for exploring intermolecular interactions in molecular crystals. J Phys Chem Lett 5(24):4249–4255. https://doi.org/10.1021/jz502271c
Turner MJ, Thomas SP, Shi MW, Jayatilaka D, Spackman MA (2015) Energy frameworks: insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem Commun 51(18):3735–3738. https://doi.org/10.1039/C4CC09074H
Mackenzie CF, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer model energies and energy frameworks: extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 4(5):575–587. https://doi.org/10.1107/S205225251700848X
Bruker (2012) APEX2, SADABS and SAINT. Bruker AXS Inc., Madison
Acknowledgements
This work was supported by Muğla Sıtkı Koçman University Research Projects Coordination Office (Grant No. 21/125/05/1).
Author information
Authors and Affiliations
Contributions
SK: Conceptualization, Methodology, Investigation, Visualization, Validation, Writing—Original Draft, Writing—Review & Editing, CT:Conceptualization, Investigation, Validation, Writing—Review & Editing, TG: Investigation, Writing—Review & Editing, TH: Methodology, Formal analysis, Investigation, Visualization, Writing—Original Draft, Writing—Review & Editing, RG: Supervision, Investigation, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kıncal, S., Topkaya, C., Göktürk, T. et al. Synthesis, Crystal Structure, Hirshfeld Surface Analysis and Interaction Energy and Energy Framework Studies of Novel Hydrazone Derivative Containing Barbituric Acid Moiety. J Chem Crystallogr 53, 81–92 (2023). https://doi.org/10.1007/s10870-022-00945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00945-1