Abstract
The synthesis and crystal structure of pyridine-3-carbaldehyde-N-ethylthiosemicarbazone (3-pytscH-NHEt) 1, and its CuI complex of stoichiometry, [CuCl(3-pytscH-NHEt)(PPh3)2] 2, studied using single crystal X-ray crystallography, are reported in this paper. Crystal data: 1, monoclinic, P21/n, a = 6.6322(3), b = 21.1200(8), c = 7.2989(3) Å; β = 91.883(4), T = 173(2), R factor = 0.0457; 2: triclinic, P-1, a = 19.3600(5), b = 20.6241(6), c = 23.8015(6) Å,α = 92.647(2), β = 104.388(2), γ = 114.377(3), R factor = 0.0662. The thio-ligand, as a neutral entity, is coordinating to Cu through its S donor atom in complex 2. It has exhibited an unusual feature of forming four independent molecules (A, B, C, D) in the unit cell, with minor differences in the bond angles / distances / torsion angles. The geometry of each molecule of 2 is distorted tetrahedral. Crystal packing, as well as Infrared, electronic absorption and proton NMR spectroscopic studies, are also reported. Copper compound 2 represents the first example of a structurally studied copper coordination compound of 3-pyridyl based thiosemicarbazones.
Graphical Abstract
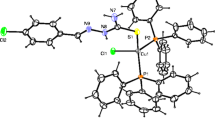
Copper(I) chloride with pyridine-3-carbaldehyde-N-ethylthiosemicarbazone and PPh3 in CH3CN yielded a copper compound, 2 (Green-Cl, blue-N; aqua-Cu, orange-S, magneta-P).






Similar content being viewed by others
References
Mydhili SP, Sireesha B, Reddy CVR (2016) J Chem Pharm Res 8:78
El-Ayaan UJ (2012) J Coord Chem 65:629
Heloisa B, Nacif WF, Teixeira LR, Reboucas JS (2002) Transition Met Chem 27:85
Refat MS, Ibrahim HK, Sowellim SZA, Soliman MH, Saeed EM (2009) J Inorg Organomet Polym 19:521
Refat MS, El-Deen IM, Zein MA, Adam AMA, Kobeasy MI (2013) Int J Electrochem Sci 8:9894
Carballo R, Casas JS, Garcia-Martinez E, Pereiras-Gabian G, Sanchez A, Abram U (2005) Cryst Eng Comm 7:113
Pereiras-Gabian G, Vazquez-Lopez EM, Braband H, Abram U (2005) Inorg Chem 44:834
Mendes IC, Teixeira LR, Lima R, Beraldo H, Speziali NL, West DX (2001) J Mol Struct 559:355
Beraldo H, Lima R, Teixeira LR, Moura AA, West DX (2001) J Mol Struct 559:99
Beraldo H, Lima R, Teixeira LR, Moura AA, West DX (2000) J Mol Struct 553:43
Lobana TS, Sharma R, Bawa G, Khanna S (2009) Coord Chem Rev 253:977
Lobana TS, Sharma R, Butcher RJ, Castineiras A, Bermejo E, Bharatam PV (2006) Inorg Chem 45:1535–1542
Lobana TS, Sharma R, Castiñeiras A, Butcher RJ (2010) Z Anorg Allg Chem 63:2698–2703
Brauer G (1965) Handbook of Preparative Chemistry, vol 2. Academic Press, New York
Andersen FE, Duca CJ, Scudi JV (1951) J Am Chem Soc 73:4967–4968
Tobias RS, Ogrins I, Nervett BA (1962) Inorg Chem 1:638
Lobana TS, Sanchez A, Casas JS, Castineiras A, Sordo J, Garcia-Tasende MS, Vazquez-Lopez EM (1997). J Chem Soc Dalton Trans. https://doi.org/10.1039/A703726K
Klayman DL, Bartosevich JF, Griffin TS, Mason CJ, Scovill JP (1979) J Med Chem 22:855
Sheldrick GM (2008) Acta Cryst A46:112
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Crystallogr 42:339
Sheldrick GM (2015) Acta Cryst C71:3
Huheey JE, Keiter EA, Keiter RL (1993) Inorganic Chemistry: Principles of Structure and Reactivity, 4th edn. Harper Collins College Publishers, New York
Funding
Financial assistance from the Council of Scientific and Industrial Research, New Delhi for SRF to Mani (Sanction letter No.: 09/254(0291)/2019-EMR-I) is gratefully acknowledged. TSL thanks The Guru Nanak Dev University for appointing Honorary Professor/ JPJ acknowledges the NSF–MRI program (Grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lobana, T.S., Kaushal, M., Kumar, A. et al. Copper(I) Derivative of Pyridine-3-carbaldehyde-N-ethylthiosemicarbazone and Triphenylphosphine—First Case of Crystal Structure Study. J Chem Crystallogr 52, 233–241 (2022). https://doi.org/10.1007/s10870-021-00911-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00911-3