Abstract
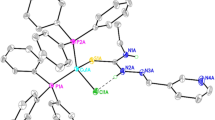
Reaction of pyrrole-2-aldehyde thiosemicarbazone (abbreviated as H 2 L, where H2 stands for the two potentially dissociable protons) with [Pd(PPh3)2Cl2] in ethanol in the presence of NEt3 afforded two complexes, [Pd(PPh3)(HLNS)Cl] and [Pd(PPh3)(LNNS)], where the thiosemicarbazone ligand is coordinated to the metal centre respectively as monoanionic N,S-donor (depicted by HLNS) and dianionic N,N,S-donor (depicted by LNNS). Similar reaction with Na2[PdCl4] afforded a bis-complex, [Pd(HLNS)2]. Crystal structures of all the three complexes have been determined. With reference to the structure of the uncoordinated thiosemicarbazone (H 2 L), the N,S-coordination mode observed in [Pd(PPh3)(HLNS)Cl] and [Pd(HLNS)2] is associated with a geometrical change around the imine bond. While the N,N,S-mode of binding observed in [Pd(PPh3)(LNNS)] takes place without any such geometrical change. All three complexes display intense absorptions in the visible and ultraviolet regions, which have been analyzed by TDDFT method.

Reaction of pyrrole-2-aldehyde thiosemicarbazone (H 2 L) with [Pd(PPh3)2Cl2] in ethanol afforded the [Pd(PPh3)(HLNS)Cl] and [Pd(PPh3)(LNNS)] complexes, and similar reaction with Na2[PdCl4] afforded the [Pd(HLNS)2] complex.





Similar content being viewed by others
References
(a) Hickey J L and Donnelly P S 2012 Coord. Chem. Rev. 256 2367; (b) Casas J S, Couce M D and Sordo J 2012 Coord. Chem. Rev. 256 3036; (c) Kalinowski J, Fattori V, Cocchi M and Williams J A G 2011Coord. Chem. Rev. 255 2401; (d) Guerchais V and Fillaut J L 2011 Coord. Chem. Rev. 255 2448; (e) Sivaramakrishna A, Clayton H S, Mogorosi M M and Moss J R 2010 Coord. Chem. Rev. 254 2904; (f) Jain V K and Jain L 2010 Coord. Chem. Rev. 2542848; (g) Berenguer J R, Lalinde E and Moreno M T 2010 Coord. Chem. Rev. 254 832; (h) Belli D, Amico D, Labella L, Marchetti F and Samaritani S 2010 Coord. Chem. Rev. 254 635; (i) Garoufis A, Hadjikakou S K and Hadjiliadis N 2009 Coord. Chem. Rev. 253 1384; (j) Lobana T S, Sharma R, Bawa G and Khanna S 2009 Coord. Chem. Rev. 253 977; (k) Quiroga A G and Ranninger C N 2004 Coord. Chem. Rev. 248 119; (l) Dilworth J R, Arnold P, Morales D, Wong Y L and Zheng Y 2002 Modern Coord. Chem. 217; (m) West D X, Liberta A E, Padhye S B, Chikate R C, Sonawane P B, Kumbhar A S and Yerande R G 1993 Coord. Chem. Rev. 123 49; (n) West D X, Padhye S B and Sonawane P B 1992 Struct. Bonding 76 1; (o) Haiduc I and Silvestru C 1990 Coord. Chem. Rev. 99 253; (p) Padhye S B and Kaffman G B 1985 Coord. Chem. Rev. 63 127; (q) M J M Campbell 1975 Coord. Chem. Rev. 15 279
(a) O’Connor M, Kellett A, McCann M, Rosair G, McNamara M, Howe O, Creaven B S, McClean S, Kia A F A, O’Shea D and Devereux M 2012 J. Med. Chem. 55 1957; (b) Skander M, Retailleau P, Bourrie B, Schio L, Mailliet P and Marinetti A 2010 J. Med. Chem. 53 2146; (c) van Rijt S H, Mukherjee A, Pizarro A M and Sadler P J 2010 J. Med. Chem. 53 840; (d) Hamberger J, Liebeke M, Kaiser M, Bracht K, Olszewski U, Zeillinger R, Hamilton G, Braun D and Bednarski P J 2009 Anti-Cancer Drugs 20 559; (e) Hrstka R, Powell D J, Kvardova V, Roubalova E, Bourougaa K, Candeias M M, Sova P, Zak F, Fåhraeus R and Vojtesek B 2008 Anti-Cancer Drugs 19 369; (f) Ma B, Djurovich P I and Thompson M E 2005 Coord. Chem. Rev. 249 1501; (g) Sova P, Mistr A, Kroutil A, Zak F, Pouckova P and Zadinova M 2005 Anti-Cancer Drugs 16 653; (h) Turánek J, Kašná A, Záluská D, Neca J, Kvardová V, Knötigová P, Horváth V, Šindlerová L, Kozubík A, Sova P, Kroutil A, žák F and Mistr A. 2004 Anti-Cancer Drugs 15537; (i) Jouad E M, Thanh X D, Bouet G, Bonneau S M and Khan A 2002 Anticancer Res. 22 1713; (j) Ferrari M B, Bisceglie F, Pelosi G, Sassi M, Tarasconi P, Cornia M, Capacchi S, Albertini R, Pinelli S 2002 J. Inorg. Biochem. 90 113; (k) Cowly A R, Dilworth J R, Donnely P S, Labisbal E and Sousa A 2002 J. Am. Chem. Soc. 124 5270; (l) Maurer R I, Blower P J, Dilworth J R, Reynolds C A, Zheng Y and Mullen G E D 2002 J. Med. Chem. 45 1420
(a) Paul P, Seth D K, Richmond M G and Bhattacharya S 2014 RSC Adv. 4 1432; (b) Dutta J and Bhattacharya S 2013 RSC Adv. 3 10707; (c) Paul P, Sengupta P and Bhattacharya S 2013 J. Organomet. Chem. 724 281; (d) Dutta J, Datta S, Seth D K and Bhattacharya S 2012 RSC Adv. 2 11751; (e) Datta S, Seth D K, Butcher R J, Gangopadhyay S, Karmakar P and Bhattacharya S 2012 Inorg. Chim. Acta 392 118; (f) Halder S, Paul P, Peng S M, Lee G H, Mukherjee A, Dutta S, Sanyal U and Bhattacharya S 2012 Polyhedron 45 177; (g) Seth D K and Bhattacharya S 2011 J. Organomet. Chem. 696 3779; (h) Datta S, Drew M G B and Bhattacharya S 2011 Indian J. Chem. Sec. A 50 1403; (i) Datta S, Seth D K, Butcher R J and Bhattacharya S 2011 Inog. Chim. Acta 377, 120; (j) Paul P, Datta S, Halder S, Acharyya R, Basuli F Butcher R J, Peng S M, Lee G H, Castineiras A, Drew M G B and Bhattacharya S 2011 J. Mol. Cat. A: Chem. 344 62; (k) Chowdhury N S, Seth D K, Drew M G B and Bhattacharya S 2011 Inog. Chim. Acta 372 183
(a) Lobana T S, Bawa G, Castineiras A and Butcher R J 2007 Inorg. Chem. Comm. 10506; (b) Alonso R, Bermejo E, Carballo R, Castiñeiras A and Pérez T 2002 J Mol. Str. 606 156
(a) Grube H L In Handbook of Preparative Inorganic Chemistry1965 Brauer G (Ed.) (London; Academic Press) 2 1584; (b) Hertley F R In The Chemistry of Platinum and Palladium1973 P L Robinson (Ed.) (London: Applied Science Publisher) 14 458
Chemical shifts are given in ppm and overlapping signals are marked with an asterisk
Meanings of the short forms within parentheses are: vs = very strong, s = strong, w = weak and vw = very weak
Frisch M J, Tracks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman Jr. J R, Montgomery J A, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalamani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa I, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskroz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A, Gaussian 03, revision D01; Gaussian Inc.: Pittsburgh, PA, 2003
Sheldrick G M SHELXS-97 and SHELXL-97, Fortran programs for crystal structure solution and refinement, University of Gottingen: Gottingen, Germany; 1997
Upon using an equimolar quantity of Na2[PdCl4] and H 2 L, the same [Pd(HLNS)2] complex is obtained in about 30% yield (based on palladium)
Acknowledgements
Financial assistance received from the Department of Science and Technology, West Bengal, India [Grant No. 746(Sanc.)/ST/P/S&T/2G-4/2013] is gratefully acknowledged. Piyali Paul thanks the Council of Scientific and Industrial Research, New Delhi, for her fellowship [Grant No. 09/096(0588)/2009-EMR-I].
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC 989560-989562 contain the supplementary crystallographic data for this paper. Results of TDDFT calculations of [Pd(PPh3)(LNNS)] (table S1) and [Pd(HLNS)2] (table S2), contour plots of selected molecular orbitals of [Pd(PPh3)(LNNS)] (figure S1) and [Pd(HLNS)2] (figure S2) are available as supplementary information.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
PAUL, P., BHATTACHARYA, S. Palladium complexes of pyrrole-2-aldehyde thiosemicarbazone: Synthesis, structure and spectral properties. J Chem Sci 126, 1547–1555 (2014). https://doi.org/10.1007/s12039-014-0699-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0699-4