Abstract
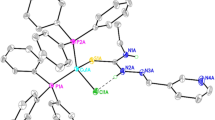
Reactions of copper(I) halides with p-chlorobenzaldehyde thiosemicarbazone (H1L) and triphenylphosphine in 1 : 1 : 2 molar ratio yielded complexes of stoichiometry, [CuX(η 1-S- H 1L)(Ph3P)2] (X = I, 1: Br, 2; Cl, 3). All the three complexes were characterized using analytical (CHNS) and spectroscopic (IR, 1H NMR) techniques. The structure of complex 3 was confirmed by X-ray crystallography. It has been found to crystallize in the triclinic system with space group P-1 and unit cell parameters: a = 10.207(5) Å, b = 13.027(5) Å, c = 16.269(5) Å, α= 100.054(5) ∘, β= 99.228(5)∘ and γ= 97.234(5)∘. This complex has distorted tetrahedral geometry with two phosphorus atoms from two triphenylphosphine ligands, thione sulfur of thiosemicarbazone ligand and chloride ion occupying the four corners of the tetrahedron. The structure of complex 3 was in contrast to sulfur-bridged dinuclear complex of copper(I) chloride with benzaldehydethiosemicarbazone, [Cu2Cl2(μ 2-S-Hbtsc)2(Ph3P)2] ⋅2H2O. The intermolecular H-bonding, Cl ⋯HC ph, 2.733 Å and π interactions, {CHph⋯π, 2.796; 2.776 Å} in this complex led to the formation of 1D chain. Two such 1D chains were cross-linked via, Cl ⋯HC ph, 2.896 Å H-bonding to form a 2D network.

p-Chlorobenzaldehyde thiosemicarbazone formed tetrahedral monomeric complex 3 with copper(I) chloride in the presence of triphenylphospine. The intermolecular H-bonding, Cl⋯HCph, 2.733 Å and π interactions, {CHph⋯π, 2.796; 2.776 Å} in this complex led to the formation of 1D chain.






Similar content being viewed by others
References
Labana T S, Sharma R, Bawa G and Khanna S 2009 Coord. Chem. Rev. 253 977
Padhye S B and Kauffman G 1985 Coord. Chem. Rev. 63 127
West D X, Padhye S B and Sonawane P B 1991 Struct. Bonding (Berlin Ger) 76 4
West D X, Liberta A E, Padhye S B, Chikate R C, Sonawane P B, Kumbhar A S and Yerande R G 1993 Coord. Chem. Rev. 123 49
Casas J S, Garcia-Tasende M S and Sordo J 2000 Coord. Chem. Rev. 209 197
Smith D R 1997 Coord. Chem. Rev. 164 575
Lobana T S, Khanna S, Sharma R, Hundal G, Sultana R, Chaudhary M, Butcher R J and Castineiras A 2008 Cryst. Growth Des. 8 1203
Mahajan R K, Kaur I and Lobana T S 2003 Talanta 59 101
Mahajan R K, Kaur I and Lobana T S 2006 Ind. J. Chem. 45A 639
Mahajan R K, Walia T P S, Sumanjit and Lobana T S 2005 Talanta 67 755
Sarma L S, Kumar J R, Kumar C J and Reddy A V 2003 Anal. Lett. 36 605
Reddy K J, Kumar J R, Ramachandraiah C, Thriveni T and Reddy A V 2007 Food Chem. 101 585
Ali M A, Khalifa M E, Ghazy S E and Hassanien M M 2002 Anal. Sci. 18 1235
Abram U, Ortner K, Gust R and Sommer K 2000 J. Chem. Soc., Dalton Trans. 735
Nomiya K, Sekino K, Ishiawa M, Honda A, Yokoyama M, Kasuga N C, Yokoyama H, Nakano S and Onodera K 2004 J. Inorg. Biochem. 98 601
Ferrari M B, Bisceglie F, Pelosi G, Tarasconi P, Albertini R, Bonati A, Lunghi P and Pinelli S 2001 Inorg. Biochem. 83 169
West D X, Ives J S, Krejci J, Salberg M M, Zumbahlen T L, Bain G A, Liberta A E, Valdes-Martinez J, Hernadez-Ortiz S and Toscano R A 1995 Polyhedron 14 2189
Garcia-Tojal J, Lezama L, Pizarro J L, Insausti M, Arriortua M I and Rojo T 1999 Polyhedron 18 3703
Lobana T S, Rekha, Butcher R J, Castineiras A, Bermejo E and Bharatam P V 2006 Inorg. Chem. 45 1535
Lobana T S, Sharma R, Castineiras A and Butcher R J 2010 Z. Anorg. Allg. Chem. 636 2698
Venkateswaran R, Balakrishna M S, Mobin S M and Tuononen H M 2007 Inorg. Chem. 46 6335
Siddiqui M M, Mague J T and Balakrishna M S 2015 Inorg. Chem. 54 6063
Lobana T S, Rani A, Jassal A K and Jasinski J P 2015 J. Chem. Sci. 127 149
Lobana T S, Kaur A, Sharma R, Bala M, Jassal A K, Duff C E and Jasinski P J 2015 J. Chem. Sci. 127 1859
Lobana T S, Khanna S, Butcher R J, Hunetr A D and Zeller M 2006 Polyhedron 25 2755
Lobana T S, Rekha, Pannu A P S, Hundal G, Butcher R J and Castineiras A 2007 Polyhedron 26 2621
Lobana T S, Rekha, Castineiras A, Sandhu B S, Bermejo E and Nishioka T 2005 J. Coord. Chem. 58 803
Lobana T S, Sharma R, Castineiras A, Hundal G and Butcher R J 2009 Inorg. Chim. Acta 362 3547
Lobana T S, Sharma R, Hundal G, Castineiras A and Butcher R J 2012 Polyhedron 47 134
Lobana T S, Kumari P, Sharma R, Castineiras A, Butcher R J, Akitsu T and Aritake Y 2011 Dalton Trans. 40 3219
Lobana T S, Khanna S, Butcher R J, Hunter A D and Zeller M 2007 Inorg. Chem. 46 5826
Lobana T S, Khanna S, Hundal G, Butcher R J and Castineiras A 2009 Polyhedron 28 3899
Brauer G 1965 In Handbook of Preparative Chemistry, Second ed. (New York: Academic Press) vol. 2
Sheldrick G M 1990 Acta Crystallogr. Sect. A 46 467
Sheldrick G M, SHELXL-97 1997P In Program for the Refinement of Crystal Structures (University of Goettingen: Goettingen, Germany)
Huheey J E, Keiter E A and Keiter R L 1993 In Inorganic Chemistry: Principals of Structure and Reactivity, 4th ed (New York: Harper Collins)
Sampath N, Mathews R and Ponnuswamy M N 2010 J. Chem. Crystallogr. 40 1110
Lobana T S, Rekha B. R J, Failes T W and Turner P 2005 J. Coord. Chem. 58 1369
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
All details have been deposited with the Cambridge Crystallographic Data Centre, CCDC for 3 is 1042089. Copies of this information can be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: 44-1223-336-033; email: deposit@ccdc.cam.ac.uk; or http://www.ccdc.cam.ac.uk). Cif file of complex 3 and PXRD patterns of complexes 1-3 are given in Supplementary Information, available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KHAN, A., SHARMA, P., RAJNIKANT et al. Synthesis and crystal structure of [chlorobis(triphenylphospino)(p-chlorobenzaldehyde thiosemicarbazone)] copper(I) complex. J Chem Sci 128, 185–191 (2016). https://doi.org/10.1007/s12039-015-1030-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-1030-8