Abstract
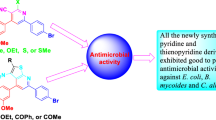
Two new quinolines derivatives where crystallized from the acetylation reaction in low temperature ; between Baylis–Hillman products which had themselves been prepared from the Meth Cohn method and catalyzed by 1,4-diazabicyclo (Fatiha et al. in J Chem Crystallogr 42:989–996, 2012) octane and silicon dioxide or in pyridine and chloroform. Chemical structures have been established by spectral techniques of FTIR, 1H NMR and X-ray single crystal in low temperature. The crystal structure of (1a) crystallizes in triclinic space group P-1, a = 7.9145(12) Å, b = 9.1412(11) Å, c = 10.7286(16) Å, α = 94.760(11)°, β = 105.988(13)° , γ = 101.818(11)°, while (2a) crystallizes in orthorhombic non- centrosymmetric space group Pna21, a = 7.7237(12) Å, b = 20.2539(28) Å, c = 8.7164(12) Å, and its cohesion was found to be assured by O–H···O and C–H···O hydrogen bonds. Antimicrobial activity (in vitro) was evaluated by Gram-positive and Gram-negative bacteria. The compounds have shown good biological activity with Gram-positive bacteria (Staphylococcus aurous and Staphylococcus coagulase-negative).
Graphical Abstract
Two new Quinoline derivatives have been prepared with good products yields; these products constitute original compounds, of great interest biological activity.









Similar content being viewed by others
References
Michael JP (1997) Nat Prod Rep 14:605
Fatiha G, Amani D, Mohammed L, Nourredine B (2012) J Chem Crystallogr 42:989–996
Levy S, Azoulay SJ (1994) Cardiovas Electrophysiol 5:635–636
Wenckebach KF (1923) JAMA 81:472–474
Roma G, Braccio MD, Grossi G, Mattioli F, Ghia H (2000) Eur J Med Chem 35:1021–1035
Bilker O, Lindo V, Panico M, Etiene AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR (1998) Nature 392:289–292
Vargas LY, Castelli MV, Kouznetsov VV, Urbina JM, Lopez SN, Sortino M, Enriz RD, Ribas JC, Zacchino S (2003) Bioorg Med Chem 11:1531–1550
Phan LT, Jian T, Chen Z, Qiu Y-L, Wang Z, Beach T, Polemeropoulos A, Or YS (2004) J Med Chem 47:2965–2968
Fang K-C, Chen Y-L, Sheu J-Y, Wang T-C, Tzeng C-C (2000) J Med Chem 43:3809–3812
Bailly C, Laine W, Baldeyrou B, De Pauw-Gillet M-C, Colson P, Houssier C, Cimanga K, Miert SV, Vlietinck AJ, Pieters L (2000) Anti-Cancer Drug Des 15:191–201
Dassonneville L, Bonjean K, De Pauw-Gillet M-C, Colson P, Houssier C, Quetin-Leclercq J, Angenot L, Ablordeppey SY (2002) Bioorg Med Chem 10:1337–1346
Khan MTH (2007) Quinoline analogs as antiangiogenic agents and telomerase inhibitors. In: Khan MTH, Gupta R (eds) Bioactive heterocycles V.R. Springer, Berlin Top Heterocycl Chem 11:213–229
Cheng X-M, Lee C, Klutchko S, Winters T, Reynolds EE, Welch KM, Flynn MA, Doherty AM (1996) Bioorg Med Chem 6:2999
Anzini M, Cappelli A, Vomero S, Giorgi G, Langer T, Hamon M, Merahi N, Emerit BM, Cagnotto A, Skorupska M, Mennini T, Pinto JC (1995) J Med Chem 38:2692
Giardina GAM, Sarau HM, Farina C, Medhurst AD, Grugni M, Raveglia LF, Schmidt DB, Rigolio R, Luttmann M, Vecchietti V, Hay DWP (1997) J Med Chem 50:1794
Holla BS, Mahalinga M, Karthikeyan MS, Akberalib PM, Shettyc NS (2006) Bioorg Med Chem 14:2040–2047
Całus S, Gondek E, Danel A, Jarosz B, Pokładko M, Kityk AV (2007) Mater Lett 61:3292–3295
Baylis AB, Hillman MED (1972) German patent, 21155113. Chem Abstr 77:34174
Meth Cohn O, Narine B, Tarnowski B (1981) J Chem Soc Perkin Trans 1:1520–1530
Narender P, Srinivas U, Gangadasu B, Biswas S, Jayathirtha Rao V (2005) Bioorg Med Chem Lett 15:5378–5381
Oxford Diffraction (2006) Xcalibur CCD system, CrysAlis Software system, Version 1.171. Oxford Diffraction Ltd., Abington
Farrugia LJ (1999) J Appl Crystallogr 32:837–838
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, De Caro L, Giacovazzo C, Polidori G, Spagna R (2005) J Appl Crystallogr 38:381–388
Sheldrick GM (1997) SHELXL97. University of Göttingen, Germany
Farrugia LJ (1997) J Appl Crystallogr 30:565
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Cryst B58:389–397
Spek AL (2003) J Appl Crystallogr 36:7–13
Grayer RJ, Harbone JB (1994) Photochemistry 73:19
Irob ON, Moo-Young M, Anderson WA (1996) Int J Pharmacol 34:87
Kalkhambkar RG, Kulkarni GM, Hwang WS, Lee CS (2008) Acta Cryst E 64:o258
Benzerka S, Bouraiou A, Bouacida S, Debache A, Belfaitah A (2008) Acta Cryst E 64:o2115–o2116
Insuasty B, Torres H, Cobo J, Lowd JN, Gilidewell C (2006) Acta Cryst C 62:O39–O41
Jasinski JP, Bucher RJ, Mayekar AN, Yathirajan HS, Narayama B, Sarojini BK (2010) J Mol Struc 980:172–181
Sayed MA, Zayed MA, Gehad GM (2010) Arab. J. Chem 3:103–113
Acknowledgments
The authors acknowledge The Tassili Programme N° 15MDU940 for the financial support. In addition, A.M and T.D acknowledge the help and advice of M.R.Y.El-Hillou (Université Larbi Ben Mhidi Oum El Bouaghi).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Djemel, T., Messai, A., Luneau, D. et al. Synthesis, Crystal Structures, Hydrogen Bonds and Antibacterial Activity of New Quinoline Derivatives. J Chem Crystallogr 45, 300–309 (2015). https://doi.org/10.1007/s10870-015-0595-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-015-0595-x