Abstract
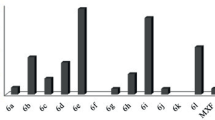
In this paper, we report the synthesis and the structure–activity relationship study of three hydrazone analogs; the Schiff base hydrazone SBH and 2, 4-dinitrophenylhydrazones H1 & H2 derived from (E)-chalcones, to identify the active fragment of each structure. This identification has been carried out following in vitro biological evaluation, which revealed that the analogs H1 and H2 showed significant antibacterial activity due to their (E)-chalcone fragments characterized by proton NMR data and demonstrated by the docked view with emphasis on the involvement of these moieties in the interaction with the DNA gyrase, and thus contributes to the pharmacophore modeling. At the same time, SBH exhibited the highest free radical DPPH scavenging power associated with hydrogen bonding and conjugated push−pull chromophores, which were elucidated by reported vibrational assignments and absorption spectra. The DFT optimizations gave rise to non-planar and distorted structures around the hydrazone group with comparable geometrical parameters. The chemical descriptors predict comparable biological activities, while the BDE necessary for the H-abstraction indicated the best antioxidant activity for the Schiff base hydrazone SBH compound.
Graphical abstract
2, 4-Dinitrophenylhydrazone analogs with (E)-chalcone and/or push-pull moieties were synthesized and characterized. The in-vitro and in-silico studies were carried out both to find the structure-activity relationship and to model the pharmacophore.

















Similar content being viewed by others
References
Liu W, Ma L, Zhang L, Chen Y, Zhang Q, Zhang H, et al. 2022 Two New Phenylhydrazone Derivatives from the Pearl River Estuary Sediment-Derived Streptomyces sp. SCSIO 40020 Mar. Drugs 20 449
Belskaya N P, Dehaen W and Bakulev V A 2010 Synthesis and properties of hydrazones bearing amide, thioamide and amidine functions Arkivoc 1 275
Nikolaevskii A, Kniga O, Khizhan E, Tikhonova G, Vinogradov V and Khizhan A 2012 Antioxidant activity of hydrazones with sterically hindered phenol fragments Russ. J. Phys. Chem. 86 1816
Vicini P, Zani F, Cozzini P and Doytchinova I 2002 Hydrazones of 1, 2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations Eur. J. Med. Chem. 37 553
Zha G-F, Leng J, Darshini N, Shubhavathi T, Vivek H, Asiri A M, et al. 2017 Synthesis, SAR and molecular docking studies of benzo [d] thiazole-hydrazones as potential antibacterial and antifungal agents Bioorg. Med. Chem. Lett. 27 3148
Yildir I, Perçiner H, Sahin M F and Abbasoglu U 1995 Hydrazones of [(2-Benzothiazolylthio) acetyl] hydrazine: Synthesis and Antimicrobial Activity Arch. Pharm. 328 547
Hameed A, Al-Rashida M, Uroos M, Abid Ali S and Khan K M 2017 Schiff bases in medicinal chemistry: a patent review (2010–2015) Expert Opin. Ther. Pat. 27 63
Maheswari R and Manjula J 2016 Vibrational spectroscopic analysis and molecular docking studies of (E)-4-methoxy-N′-(4-methylbenzylidene) benzohydrazide by DFT J. Mol. Struct. 1115 144
Khamaysa O M A, Selatnia I, Zeghache H, Lgaz H, Sid A, Chung I-M, et al. 2020 Enhanced corrosion inhibition of carbon steel in HCl solution by a newly synthesized hydrazone derivative: Mechanism exploration from electrochemical, XPS, and computational studies J. Mol. Liq. 315 113805
Khamaysa O M A, Selatnia I, Lgaz H, Sid A, Lee H-S, Zeghache H, et al. 2021 Hydrazone-based green corrosion inhibitors for API grade carbon steel in HCl: Insights from electrochemical, XPS, and computational studies Colloids Surf. A 626 127047
Al Zoubi W 2013 Biological activities of Schiff bases and their complexes: a review of recent works Int. J. Org. Chem. 3 73
Aggarwal S, Paliwal D, Kaushik D, Gupta G K and Kumar A 2018 Pyrazole Schiff base hybrids as anti-malarial agents: synthesis, in vitro screening and computational study Comb. Chem. High Throughput Screen. 21 194
Sridhar S K, Pandeya S N, Stables J P and Ramesh A 2002 Anticonvulsant activity of hydrazones, Schiff and Mannich bases of isatin derivatives Eur. J. Pharm. Sci. 16 129
Xu J, Zhou T, Xu Z-Q, Gu X-N, Wu W-N, Chen H, et al. 2017 Synthesis, crystal structures and antitumor activities of copper (II) complexes with a 2-acetylpyrazine isonicotinoyl hydrazone ligand J. Mol. Struct. 1128 448
Ali S M M, Azad M A K, Jesmin M, Ahsan S, Rahman M M, Khanam J A, et al. 2012 In vivo anticancer activity of vanillin semicarbazone Asian Pac. J. Trop. Biomed. 2 438
Shockravi A, Sadeghpour M and Olyaei A 2010 Simple and Efficient Procedure for the Synthesis of Symmetrical Bis-Schiff Bases of 5, 5′-Methylenebis (2-aminothiazole) Under Solvent-Free Conditions Synth. Commun. 40 2531
Saouli S, Selatnia I, Zouchoune B, Sid A, Zendaoui S M, Bensouici C and Bendeif E-E 2020 Synthesis, spectroscopic characterization, crystal structure, DFT studies and biological activities of new hydrazone derivative: 1-(2,5-bis((E)-4-isopropylbenzylidene) cyclopentylidene)-2-(2,4-dinitrophenyl) hydrazine J. Mol. Struct. 1213 128203
Zouchoune B 2020 How the ascorbic acid and hesperidin do improve the biological activities of the cinnamon: Theoretical investigation Struct. Chem. 31 2333
Zouchoune B 2021 Theoretical investigation on the biological activities of ginger and some of its combinations: an overview of the antioxidant activity Struct. Chem. 32 1659
Dammene Debbih O, Sid A, Bouchene R, Bouacida S, Mazouz W and Gherraf N 2018 Two hydrazones derived from 1-aryl-3-(p-substituted phenyl) prop-2-en-1-one: synthesis, crystal structure, Hirshfeld surface analysis and in vitro biological properties Acta Crystallogr. C Struct. Chem. 74 703
Rosca I, Petrovici A R, Brebu M, Stoica I, Minea B and Marangoci N 2016 An original method for producing acetaldehyde and diacetyl by yeast fermentation Braz. J. Microbiol. 47 949
Humphries R M, Hindler J A, Shaffer K and Campeau S A 2019 Evaluation of ciprofloxacin and levofloxacin disk diffusion and Etest using the 2019 Enterobacteriaceae CLSI breakpoints J. Clin. Microbiol. 57 e01797
Constantin S, Lupascu F G, Apotrosoaei M, Vasincu I M, Lupascu D, Buron F, et al. 2017 Synthesis and biological evaluation of the new 1, 3-dimethylxanthine derivatives with thiazolidine-4-one scaffold Chem. Cent. J. 11 12
Sánchez-Moreno C, Larrauri J A and Saura-Calixto F 1998 A procedure to measure the antiradical efficiency of polyphenols J. Sci. Food Agric. 76 270
Bentabet N, Boucherit-Otmani Z and Boucherit K 2014 Composition chimique et activité antioxydante d’extraits organiques des racines de Fredolia aretioides de la région de Béchar en Algérie Phytothérapie 12 364
ADF2016.01 Version, Theoretical Chemistry, Vrije Universiteit: Amsterdam. The Netherlands, SCM
Baerends E, Ellis D and Ros P 1973 Self-consistent molecular Hartree—Fock—Slater calculations I. The computational procedure Chem. Phys. 2 41
Te Velde G and Baerends E 1992 Numerical integration for polyatomic systems J. Comput. Phys. 99 84
Fonseca Guerra C, Snijders J, Te Velde Gt and Baerends E J 1998 Towards an order-N DFT method Theor. Chem. Acc. 99 391
Bickelhaupt F M and Baerends E J 2000 Kohn-Sham density functional theory: predicting and understanding chemistry Rev. Comput. Chem. 15 1
Te Velde Gt, Bickelhaupt F M, Baerends E J, Fonseca Guerra C, van Gisbergen S J, Snijders J G and Ziegler T 2001 Chemistry with ADF J. Comput. Chem. 22 931
Vosko S H, Wilk L and Nusair M 1980 Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis Can. J. Phys. 58 1200
Becke A D 1993 Becke’s three parameter hybrid method using the LYP correlation functional J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density Phys. Rev. B 37 785
Versluis L and Ziegler T 1988 The determination of molecular structures by density functional theory. The evaluation of analytical energy gradients by numerical integration J. Chem. Phys. 88 322
Fan L and Ziegler T 1992 Application of density functional theory to infrared absorption intensity calculations on main group molecules J. Chem. Phys. 96 9005
Fan L and Ziegler T 1992 Application of density functional theory to infrared absorption intensity calculations on transition-metal carbonyls J. Phys. Chem. 96 6937
DiLabio G, Pratt D, LoFaro A and Wright J S 1999 Theoretical study of X− H bond energetics (X = C, N, O, S): Application to substituent effects, gas phase acidities, and redox potentials J. Phys. Chem. A 103 1653
Feng Y, Liu L, Wang J-T, Huang H and Guo Q-X 2003 Assessment of experimental bond dissociation energies using composite ab initio methods and evaluation of the performances of density functional methods in the calculation of bond dissociation energies J. Chem. Inf. Comput. Sci. 43 2005
Klamt A and Schüürmann G 1993 COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient J. Chem. Soc. Perkin Trans. 2 799
Trott O and Olson A J 2010 AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading J. Comput. Chem. 31 455
Tai W, Lu T, Yuan H, Wang F, Liu H, Lu S, et al. 2012 Pharmacophore modeling and virtual screening studies to identify new c-Met inhibitors J. Mol. Model. 18 3087
OriginPro 9.0 SR2 software 2013 OriginLab Corporation, Northampton, MA, USA, April 2013
Enrique S 2018 MestReNova [software]. Version 12.0.2-20910, Mestrelab Research, Santiago de Compostela, Spain
Sundaraganesan N, Kalaichelvan S, Meganathan C, Joshua B D and Cornard J 2008 FT-IR, FT-Raman spectra and ab initio HF and DFT calculations of 4-N, N′-dimethylamino pyridine Spectrochim. Acta - Part A 71 898
Hamidian K, Irandoust M, Rafiee E and Joshaghani M 2012 Synthesis, characterization, and tautomeric properties of some azo-azomethine compounds Zeitschrift für Naturforschung B 67 159
Yang P, Zhao J, Zhang L, Li L and Zhu Z 2015 Intramolecular hydrogen bonds quench photoluminescence and enhance photocatalytic activity of carbon nanodots Chem. Eur. J. 21 8561
Olszowy M 2019 What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 144 135
Wang L, Yang Q, Li Y, Wang S, Yang F and Zhao X 2021 How the functional group substitution and solvent effects affect the antioxidant activity of (+)-catechin? J. Mol. Liq. 327 114818
Masoud M S, Ali A E, Shaker M A and Ghani M A 2005 Solvent and substituent effects on spectroscopical changes of some diazoaminobenzene derivatives Spectrochim. Acta 61 3102
Thavasi V, Bettens R P A and Leong L P 2009 Temperature and solvent effects on radical scavenging ability of phenols J. Phys. Chem. A 113 3068
Cuvelier M-E, Richard H and Berset C 1992 Comparison of the antioxidative activity of some acid-phenols: structure-activity relationship Biosci. Biotechnol. Biochem. 56 324
Subramanian M, Vanangamudi G and Thirunarayanan G 2013 Hydroxyapatite catalyzed aldol condensation: Synthesis, spectral linearity, antimicrobial and insect antifeedant activities of some 2, 5-dimethyl-3-furyl chalcones Spectrochim. Acta – Part A 110 116
Dusso D, Ramirez C, Parise A, Lanza P, Vera D M, Chesta C, et al. 2019 Synthesis of new cyano-substituted analogues of Tröger’s bases from bromo-derivatives. A stereochemical dependence of long-range (nJHH, n = 4, 5, and 6) proton–proton and proton–carbon (nJCH, n = 1, 2, 3, 4, and 5) coupling constants of these compounds Magn. Reson. Chem. 57 423
Souza M A D, Rodrigues L G, Rocha J E, de Freitas T S, Bandeira P N, Marinho M M, Nunes da Rocha M, Marinho E S, Honorato Barreto A C and Coutinho H D M 2023 Synthesis, structural, characterization, antibacterial and antibiotic modifying activity, ADMET study, molecular docking and dynamics of chalcone (E)-1-(4-aminophenyl)-3-(4-nitrophenyl) prop-2-en-1-one in strains of Staphylococcus aureus carrying NorA and MepA efflux pumps J. Biomol. Struct. Dyn. 1
Naili N and Zouchoune B 2018 Structural diversity of homobinuclear transition metal complexes of the phenazine ligand: theoretical investigation Struct. Chem. 29 725
Nemdili H, Zouchoune B, Zendaoui M S and Ferhati A 2019 Structural, bonding and redox properties of 34-electron bimetallic complexes and their oxidized 32-and 33-electron and reduced 35-and 36-electron derivatives containing the indenyl fused π-system: A DFT overview Polyhedron 160 219
Farah S, Bouchakri N, Zendaoui S-M, Saillard J-Y and Zouchoune B 2010 Electronic structure of bis-azepine transition-metal complexes: a DFT investigation J. Mol. Struct. THEOCHEM 953 143
Farah S, Ababsa S, Benhamada N and Zouchoune B 2010 Theoretical investigation of the coordination of dibenzazepine to transition-metal complexes: a DFT study Polyhedron 29 2722
Bouchakri N, Benmachiche A and Zouchoune B 2011 Bonding analysis and electronic structure of transition metal–benzoquinoline complexes: A theoretical study Polyhedron 30 2644
Mansouri L and Zouchoune B 2015 Substitution effects and electronic properties of the azo dye (1-phenylazo-2-naphthol) species: a TD-DFT electronic spectra investigation Can. J. Chem. 93 509
Zouchoune B and Mansouri L 2019 Electronic structure and UV–Vis spectra simulation of square planar Bis (1-(4-methylphenylazo)-2-naphtol)-Transition metal complexes [M (L) 2] x (M = Ni, Pd, Pt, Cu, Ag, and x\(=\)− 1, 0,+ 1): DFT and TD-DFT study Struct. Chem. 30 691
Zendaoui S-M and Zouchoune B 2016 Coordination chemistry of mixed M (benzene)(cyclopendadienyl) sandwich complexes: electronic properties and bonding analysis New J. Chem. 40 2554
Bensalem N and Zouchoune B 2016 Coordination capabilities of anthracene ligand in binuclear sandwich complexes: DFT investigation Struct. Chem. 27 1781
Wiberg K B 1968 Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane Tetrahedron 24 1083
Weinhold F and Landis C R 2005 Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective (Cambridge University Press: Cambridge)
Weinhold F and Glendening E D 2001 NBO 5.0 program manual: natural bond orbital analysis programs. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison, WI 53706
Li X-H, Liu X-R and Zhang X-Z 2011 Molecular structure and vibrational spectra of three substituted 4-thioflavones by density functional theory and ab initio Hartree-Fock calculations Spectrochim. Acta 78 528
Padmaja L, Ravikumar C, Sajan D, Hubert Joe I, Jayakumar V, Pettit G and Faurskov Nielsen O 2009 Density functional study on the structural conformations and intramolecular charge transfer from the vibrational spectra of the anticancer drug combretastatin-A2 J. Raman Spectrosc. 40 419
Roy D, Sarkar U, Chattaraj P, Mitra A, Padmanabhan J, Parthasarathi R, et al. 2006 Analyzing toxicity through electrophilicity Mol. Diver. 10 119
Roy D, Pal N, Mitra A, Bultinck P, Parthasarathi R, Subramanian V and Chattaraj P 2007 An atom counting strategy towards analyzing the biological activity of sex hormones Eur. J. Med. Chem. 42 1365
Pearson R G 1987 Recent advances in the concept of hard and soft acids and bases J. Chem. Educ. 64 561
Parr R G and Yang W 1989 Density-functional theory of atoms and molecules Oxford (Oxford University Press: Oxford)
Parr R G, Szentpaly L V and Liu S 1999 Electrophilicity Index J. Am. Chem. Soc. 121 1922
Chattaraj P K, Maiti B and Sarkar U 2003 Philicity: A unified treatment of chemical reactivity and selectivity J. Phys. Chem. A 107 4973
Chattaraj P K, Sarkar U and Roy D R 2006 Electrophilicity index Chem. Rev. 106 2065
Dan W and Dai J 2020 Recent developments of chalcones as potential antibacterial agents in medicinal chemistry Eur. J. Med. Chem. 187 111980
Farhadi F, Khameneh B, Iranshahi M and Iranshahy M 2019 Antibacterial activity of flavonoids and their structure–activity relationship: An update review Phytotherapy Res. 33 13
Nowakowska Z, Kędzia B and Schroeder G 2008 Synthesis, physicochemical properties and antimicrobial evaluation of new (E)-chalcones Eur. J. Med. Chem. 43 707
Nowakowska Z 2007 A review of anti-infective and anti-inflammatory chalcones Eur. J. Med. Chem. 42 125
Chen J, Yang J, Ma L, Li J, Shahzad N and Kim C K 2020 Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids Sci. Rep. 10 1
Ouyang X, Li X, Liu J, Liu Y, Xie Y, Du Z, et al. 2020 Structure–activity relationship and mechanism of four monostilbenes with respect to ferroptosis inhibition RSC Adv. 10 31171
Wright J S, Johnson E R and DiLabio G A 2001 Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants J. Am. Chem. Soc. 123 1173
Litwinienko G and Ingold K 2003 Abnormal solvent effects on hydrogen atom abstractions. 1. The reactions of phenols with 2, 2-diphenyl-1-picrylhydrazyl (dpph•) in alcohols J. Org. Chem. 68 3433
Leopoldini M, Marino T, Russo N and Toscano M 2004 Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism J. Phys. Chem. A 108 4916
Leopoldini M, Marino T, Russo N and Toscano M 2004 Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent Theor. Chem. Acc. 111 210
Leopoldini M, Russo N, Chiodo S and Toscano M 2006 Iron chelation by the powerful antioxidant flavonoid quercetin J. Agric. Food Chem. 54 6343
Charlton N C, Mastyugin M, Török B and Török M J M 2023 Structural features of small molecule antioxidants and strategic modifications to improve potential bioactivity Molecules 28 1057
Peerannawar S, Horton W, Kokel A, Török F, Török M and Török B 2017 Theoretical and experimental analysis of the antioxidant features of diarylhydrazones Struct. Chem. 28 391
Ayoup M S, Rabee A R, Abdel-Hamid H, Harras M F, El Menofy N G and Ismail M M 2022 Exploration of nitroaromatic antibiotics via Sanger’s reagent: Synthesis, in silico, and antimicrobial evaluation ACS Omega 7 5254
Coleman J P and Smith C J 2007 Microbial Nucleic Acid and Protein Synthesis S J Enna and David B Bylund (Eds) xPharm: The Comprehensive Pharmacology Reference (Elsevier) p. 1
Spížek J, Novotná J, Řezanka T and Demain A L 2010 Do we need new antibiotics? The search for new targets and new compounds J. Ind. Microbiol. Biotechnol. 37 1241
Clark D E 2008 What has virtual screening ever done for drug discovery? Expert Opin. Drug Discov. 3 841
Qureshi M A, Akbar M, Amir M and Javed S 2023 Molecular interactions of esculin with bovine serum albumin and recognition of binding sites with spectroscopy and molecular docking J. Biomol. Struct. Dyn. 41 2630
Lipinski C A, Lombardo F, Dominy B W and Feeney P J 1997 Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings Adv. Drug Deliv. Rev. 23 3
Borkar M, Prabhu A, Kanugo A and Gautam R K 2023 Pharmacophore modeling, in Computational Approaches in Drug Discovery, Development and Systems Pharmacology Elsevier 159
Khedkar S A, Malde A K, Coutinho E C and Srivastava S 2007 Pharmacophore modeling in drug discovery and development: an overview Med. Chem. 3 187
Lengerli D, Ibis K, Nural Y and Banoglu E 2022 The 1, 2, 3-triazole ‘all-in-one’ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool Expert Opin. Drug Discov. 17 1209
Acknowledgements
The authors are grateful to DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue on Interplay of Structure and Dynamics in Reaction Pathways, Chemical Reactivity and Biological Systems
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dammene Debbih, O., Mazouz, W., Benslama, O. et al. Hydrazone analogs as DNA gyrase inhibitors and antioxidant agents: Structure-activity relationship and pharmacophore modeling. J Chem Sci 136, 32 (2024). https://doi.org/10.1007/s12039-024-02264-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-024-02264-8