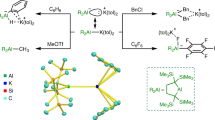
The reactions of germanium dichloride and dimethyl aluminium chloride with 2,2-dipyridylamine (dpa) affords germanium and organo-aluminium complexes. Structural characterization reveals that the preferential coordination site of both germanium and aluminium is within the sterically protected pyridyl, (pyridyl = C5H5N = py), pocket. Py2NGeCl, compound (1), crystallizes in the triclinic space group, P-1, with cell coordinates; a=8.2956(11), b=9.9938(13), c=12.6517(17), α=92.033(2)°, β=92.834(2)°, γ=91.685(2)°. The aluminium complex, Py2NAlMe2·Al(Me)Cl, (2), crystallizes in the orthorhombic space group, Pna2(1), with cell parameters: a=13.3707(10), b=10.8706(8), c=16.1306(12). The X-ray analysis of the side products of these reactions, the lithium halide adducts are also reported.





Similar content being viewed by others
References
(a) Dillingham, M.D.; Schauer, S.J.; Byers-Hill, J.; Pennington, W.H.; Robinson, G.H. J. Coord. Chem. 1994, 31(4), 283; (b) Robinson, G.H. Coord. Chem. Alum. 1993, 57; (c) Panella, A.; Pons, J.; Garcia-Anton, J.; Solans, X.; Font-Bardia, M.; Ros, J. Inorg. Chim. Acta. 2006, 359(8), 2343; (d) Mohamadou, A.; Gerard, C. J. Chem. Soc., Dalton Trans. 2001, 22; (e) Reger, D.L.; Collins, J.E.; Matthews, M.A.; Rheingold, A.L.; Liable-Sands, L.M.; Guzei, L.A. Inorg. Chem. 1997, 36(27); (f) Liang, L.C.; Huang, M.H.; Hung, C.-H. Inorg. Chem. 2004, 43(6), 2166.
(a) Veith, M. Top. Organomet. Chem. 2005, 9, 81–100, (Precursor Chemistry of Advanced Materials: CVD, ALD and Nanoparticles); (b) Itsuki, A. Jpn. Kokai Tokkyo Koho 2005, 8; (c) Kraus, B.D.; Lane, R.H., U.S., 2002, 8 pp.; (d) Shin, H-K. Eur. Pat. Appl. 2000, 22 pp.; (e) Passarelli, V.; Carta, G.; Rossetto, G.; Zanella, P. Dalton Trans. 2003, 1284.
(a) Clėrac, R.; Cotton, F.A.; Daniels, L.M.; Dunbar, K.R.; Murillo, C.A.; Wang, X. Inorg. Chem., 2001, 40, 1256–1264; (b) Cotton, F.A.; Daniels, L.M.; Gordon IV, G.T.; Murrillo, C.A. J. Am. Chem. Soc. 1997, 119, 10377; (c) Fandos, R.; Hernández, C.; Otero, A.; Rodriguez, A.; Ruiz, M. J. Organomet. Chem. 2005, 690, 4828.
Pfeiffer, M.; Murso, A.; Mahalakshmi, L.; Moigno, D.; Kiefer, W.; Stalke, D. Eur. J. Inorg. Chem. 2002, (12), 3222; (b) Ashenhurst, J.; Brancaleon, L.; Gao, S.; Liu, W.; Schmider, H.; Wang, S.; Wu, G.; Wu, Q.G. Organometallics 1998, 17(24), 5334; (c) Gornitzka, H.; Stalke, D. Eur. J. Inorg. Chem. 1998, (3), 311; (d) Wang, X.M.; Sun, H.-S.; You, X.-Z.; Huang, X.-Y. Polyhedron 1996, 15(20), 3543.
Sheldrick, G.M. SHELXS-97, Program for the solution of crystal structures, Germany: University of Göttingen, 1997.
Sheldrick, G.M. SHELXL-97, Program for the refinement of crystal structures.
Cui, C.; Roesky, H.W.; Noltemeyer, M.; Lappert, M.F.; Schmidt, H-G.; Hao, H. Organometallics 1999, 18, 2256.
(a) Hill, J.B.; Eng, S.J.; Pennington, W.T.; Robinson, G.H. J. Organomet. Chem. 1993, 445, 11; (b) Hill, J.B.; Talley, T.A.; Pennington, W.T.; Robinson, G.H. J. Chem. Crystallogr. 1994, 24, 61; (c) Shukla, P.; Gordon, J.C.; Cowley, A.H.; Jones, J.N. Inorg. Chim. Acta 2005, 358, 4407; (d) Haage, K.; Starowieyski, K.B.; Chwojnowski, A. J. Organomet. Chem. 1979, 174(2), 149; (e) Kim, S.J.; Yang, N.; Kim, D.-H.; Kang, S.O.; Ko, J. Organometallics 2000, 19(20), 4036; (f) Tyron, E.K.; Schauer, S.J.; Lake, C.H.; Watkins, C.L.; Krannich, L.K. J. Organomet. Chem. 1999, 585(2), 266.
Self, M.F.; Pennington, W.T.; Laske, J.A.; Robinson, G.H. Organometallics 1991, 10, 36.
(a) Engelhardt, L.M.; Jacobsen, G.E.; White, A.H.; Raston, C.L. Inorg. Chem. 1991, 30(21), 3978; (b) Piers, W.E.; Bunel, E.E.; Bercaw, J.E. J. Organomet. Chem. 1991, 407(1), 51.
Acknowledgment
TCU, TCU-RCAF and the Welch Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Crystallographic data for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 611221 for compound (1), CCDC No. 611223, compound (2), CCDC No. 611228, compound (3) and CCDC No. 611227 for compound (4).
Copies of this information may be obtained free of charge from: The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ UK, Fax. (int code) +44(1223)336-033 or Email: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Gushwa, A.F., Richards, A.F. The synthesis and characterization of germanium dichloride and dimethyl aluminium chloride derivatives of the 2,2-dipyridylamine ligand. J Chem Crystallogr 36, 851–856 (2006). https://doi.org/10.1007/s10870-006-9139-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-006-9139-8