Abstract
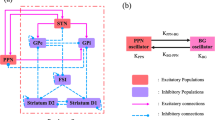
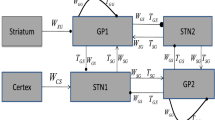
Excessive neural synchronization of neural populations in the beta (β) frequency range (12–35 Hz) is intimately related to the symptoms of hypokinesia in Parkinson’s disease (PD). Studies have shown that delayed feedback stimulation strategies can interrupt excessive neural synchronization and effectively alleviate symptoms associated with PD dyskinesia. Work on optimizing delayed feedback algorithms continues to progress, yet it remains challenging to further improve the inhibitory effect with reduced energy expenditure. Therefore, we first established a neural mass model of the cortex-basal ganglia-thalamus-pedunculopontine nucleus (CBGTh-PPN) closed-loop system, which can reflect the internal properties of cortical and basal ganglia neurons and their intrinsic connections with thalamic and pedunculopontine nucleus neurons. Second, the inhibitory effects of three delayed feedback schemes based on the external globus pallidum (GPe) on β oscillations were investigated separately and compared with those based on the subthalamic nucleus (STN) only. Our results show that all four delayed feedback schemes achieve effective suppression of pathological β oscillations when using the linear delayed feedback algorithm. The comparison revealed that the three GPe-based delayed feedback stimulation strategies were able to have a greater range of oscillation suppression with reduced energy consumption, thus improving control performance effectively, suggesting that they may be more effective for the relief of Parkinson’s motor symptoms in practical applications.










Similar content being viewed by others
Data availability
Data available on request.
References
Cabezudo, D., Baekelandt, V., Lobbestael, E.: Multiple-hit hypothesis in Parkinson’s disease: LRRK2 and inflammation. Front. Neurosci. 14, 376 (2020). https://doi.org/10.3389/fnins.2020.00376
Stefani, A., Trendafilov, V., Liguori, C., et al.: Subthalamic nucleus deep brain stimulation on motor-symptoms of Parkinson’s disease: focus on neurochemistry. Prog. Neurobiol. 151, 157–174 (2017). https://doi.org/10.1016/j.pneurobio.2017.01.003
Guo, Y., Rubin, J.E.: Multi-site stimulation of subthalamic nucleus diminishes thalamocortical relay errors in a biophysical network model. Neural Netw. 24(6), 602–616 (2011). https://doi.org/10.1016/j.neunet.2011.03.010
Fountas, Z., Shanahan, M.: The role of cortical oscillations in a spiking neural network model of the basal ganglia. PLoS ONE 12(12), e0189109 (2017). https://doi.org/10.1371/journal.pone.0189109
Liu, C., Wang, J., Li, H., et al.: Modeling and analysis of beta oscillations in the basal ganglia. IEEE Trans. Neural Netw. Learn. Syst. 29(5), 1864–1875 (2018). https://doi.org/10.1109/TNNLS.2017.2688426
Mallet, N., Pogosyan, A., Sharott, A., et al.: Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 28(18), 4795–4806 (2008). https://doi.org/10.1523/JNEUROSCI.0123-08.2008
Rodriguez-Oroz, M.C., Obeso, J.A., Lang, A.E., et al.: Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain 128(10), 2240–2249 (2005). https://doi.org/10.1093/brain/awh571
Deuschl, G., Schade-Brittinger, C., Krack, P., et al.: A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355(9), 896–908 (2006). https://doi.org/10.1056/NEJMoa060281
deSouza, R.M., Akram, H., Low, H.L., et al.: The timing of deep brain stimulation for Parkinson disease in the UK from 1997 to 2012. Eur. J. Neurol. 22(10), 1415–1417 (2015). https://doi.org/10.1111/ene.12795
Tinkhauser, G., Pogosyan, A., Little, S., et al.: The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 140(4), 1053–1067 (2017). https://doi.org/10.1093/brain/awx010
Yu, Y., Hao, Y., Wang, Q.: Model-based optimized phase-deviation deep brain stimulation for Parkinson’s disease. Neural Netw. 122, 308–319 (2020). https://doi.org/10.1016/j.neunet.2019.11.001
Dorval, A.D., Grill, W.M.: Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J. Neurophysiol. 111(10), 1949–1959 (2014). https://doi.org/10.1152/jn.00713.2013
Lu, C., Amundsen Huffmaster, S.L., Louie, K.H., et al.: Pallidal oscillation dynamics following cessation of deep brain stimulation in Parkinson’s disease. Mov. Disord. 35(9), 1697–1698 (2020). https://doi.org/10.1002/mds.28227
Harmsen, I.E., Elias, G.J.B., Beyn, M.E., et al.: Clinical trials for deep brain stimulation: current state of affairs. Brain Stimul. 13(2), 378–385 (2020). https://doi.org/10.1016/j.brs.2019.11.008
Wang, K., Wang, J., Zhu, Y., et al.: Adaptive closed-loop control strategy inhibiting pathological basal ganglia oscillations. Biomed. Signal Process. Control 77, 103776 (2022). https://doi.org/10.1016/j.bspc.2022.103776
Pyragas, K.: Continuous control of chaos by self-controlling feedback. Phys. Lett. A 170(6), 421–428 (1992). https://doi.org/10.1016/0375-9601(92)90745-8
Popovych, O.V., Hauptmann, C., Tass, P.A.: Control of neuronal synchrony by nonlinear delayed feedback. Biol. Cybern. 95(1), 69–85 (2006). https://doi.org/10.1007/s00422-006-0066-8
Lin, W., Pu, Y., Guo, Y., et al.: Oscillation suppression and synchronization: frequencies determine the role of control with time delays. Europhys. Lett. 102(2), 20003 (2013). https://doi.org/10.1209/0295-5075/102/20003
Wedgwood, K.C.A., Słowiński, P., Manson, J., et al.: Robust spike timing in an excitable cell with delayed feedback. J. Royal Soc. Interface 18(177), 20210029 (2021). https://doi.org/10.1098/rsif.2021.0029
Rosenblum, M., Pikovsky, A.: Delayed feedback control of collective synchrony: an approach to suppression of pathological brain rhythms. Phys. Rev. E 70(4), 041904 (2004). https://doi.org/10.1103/PhysRevE.70.041904
Hövel, P., Schöll, E.: Control of unstable steady states by time-delayed feedback methods. Phys. Rev. E 72(4), 046203 (2005). https://doi.org/10.1103/PhysRevE.72.046203
Popovych, O., Lysyansky, B., Rosenblum, M., et al.: Pulsatile desynchronizing delayed feedback for closed-loop deep brain stimulation. PLoS ONE 12(3), e0173363 (2017). https://doi.org/10.1371/journal.pone.0173363
Popovych, O.V., Tass, P.A.: Multisite delayed feedback for electrical brain stimulation. Front. Physiol. 9, 46 (2018). https://doi.org/10.3389/fphys.2018.00046
Daneshzand, M., Faezipour, M., Barkana, B.D.: Delayed feedback frequency adjustment for deep brain stimulation of subthalamic nucleus oscillations. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2194–2197 (2018). https://doi.org/10.1109/EMBC.2018.8512652
Popovych, O.V., Tass, P.A.: Adaptive delivery of continuous and delayed feedback deep brain stimulation—a computational study. Sci. Rep. 9(1), 10585 (2019). https://doi.org/10.1038/s41598-019-47036-4
Liu, C., Zhou, C., Wang, J., et al.: Delayed feedback-based suppression of pathological oscillations in a neural mass model. IEEE Trans. Cybern. 51(10), 5046–5056 (2021). https://doi.org/10.1109/TCYB.2019.2923317
Rowe, D.L., Robinson, P.A., Rennie, C.J.: Estimation of neurophysiological parameters from the waking EEG using a biophysical model of brain dynamics. J. Theor. Biol. 231(3), 413–433 (2004). https://doi.org/10.1016/j.jtbi.2004.07.004
Engblom, S., Wilson, D.B., Baker, R.E.: Scalable population-level modelling of biological cells incorporating mechanics and kinetics in continuous time. Royal Soc. Open Sci. 5(8), 180379 (2018). https://doi.org/10.1098/rsos.180379
Ahmadizadeh, S., Karoly, P.J., Nešić, D., et al.: Bifurcation analysis of two coupled Jansen-Rit neural mass models. PLoS ONE 13(3), e0192842 (2018). https://doi.org/10.1371/journal.pone.0192842
Köksal Ersöz, E., Modolo, J., Bartolomei, F., et al.: Neural mass modeling of slow-fast dynamics of seizure initiation and abortion. PLoS Comput. Biol. 16(11), e1008430 (2020). https://doi.org/10.1371/journal.pcbi.1008430
Luyao Yan, A., Honghui Zhang, B., Zhongkui Sun, C., et al.: Mechanism analysis for excitatory interneurons dominating poly-spike wave and optimization of electrical stimulation. Chaos An. Interdiscip. J. Nonlinear Sci. 32(3), 033110 (2022). https://doi.org/10.1063/5.0076439
Ford, B., Holmes, C.J., Mainville, L., et al.: GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J. Comp. Neurol. 363(2), 177–196 (1995). https://doi.org/10.1002/cne.903630203
Lee, M.S., Rinne, J.O., Marsden, C.D.: The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med. J. 41(2), 167 (2000). https://doi.org/10.3349/ymj.2000.41.2.167
Mena-Segovia, J., Bolam, J.P., Magill, P.J.: Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 27(10), 585–588 (2004). https://doi.org/10.1016/j.tins.2004.07.009
Ye, M., Hayar, A., Strotman, B., et al.: Cholinergic modulation of fast inhibitory and excitatory transmission to pedunculopontine thalamic projecting neurons. J. Neurophysiol. 103(5), 2417–2432 (2010). https://doi.org/10.1152/jn.01143.2009
Liu, C., Zhou, C., Wang, J., et al.: The role of coupling connections in a model of the cortico-basal ganglia-thalamocortical neural loop for the generation of beta oscillations. Neural Netw. 123, 381–392 (2020). https://doi.org/10.1016/j.neunet.2019.12.021
Moran, R.J., Mallet, N., Litvak, V., et al.: Alterations in brain connectivity underlying beta oscillations in parkinsonism. PLoS Comput. Biol. 7(8), e1002124 (2011). https://doi.org/10.1371/journal.pcbi.1002124
Corbit, V.L., Whalen, T.C., Zitelli, K.T., et al.: Pallidostriatal projections promote oscillations in a dopamine-depleted biophysical network model. J. Neurosci. 36(20), 5556–5571 (2016). https://doi.org/10.1523/JNEUROSCI.0339-16.2016
Madadi Asl, M., Asadi, A., Enayati, J., et al.: Inhibitory spike-timing-dependent plasticity can account for pathological strengthening of pallido-subthalamic synapses in Parkinson’s disease. Front. Physiol. 13, 915626 (2022). https://doi.org/10.3389/fphys.2022.915626
Lee, K., Masmanidis, S. C.: Aberrant features of in vivo striatal dynamics in Parkinson’s disease. J. Neurosci. Res. 97(12), 1678–1688 (2019). https://doi.org/10.1002/jnr.24519
Brown, P., Oliviero, A., Mazzone, P., et al.: Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. 21(3), 1033–1038 (2001). https://doi.org/10.1523/JNEUROSCI.21-03-01033.2001
David, O., Friston, K.J.: A neural mass model for MEG/EEG. Neuroimage 20(3), 1743–1755 (2003). https://doi.org/10.1016/j.neuroimage.2003.07.015
Jansen, B.H., Rit, V.G.: Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol. Cybern. 73(4), 357–366 (1995). https://doi.org/10.1007/BF00199471
Yu, Y., Zhang, H., Zhang, L., et al.: Dynamical role of pedunculopntine nucleus stimulation on controlling Parkinson’s disease. Physica A 525, 834–848 (2019). https://doi.org/10.1016/j.physa.2019.04.016
Oswal, A., Cao, C., Yeh, C.-H., et al.: Neural signatures of hyperdirect pathway activity in Parkinson’s disease. Nat. Commun. 12(1), 5185 (2021). https://doi.org/10.1038/s41467-021-25366-0
Wei, W., Zhang, Z., Chen, N., et al.: On disturbance rejection control of the epileptiform spikes. Cogn. Neurodyn. 16(2), 425–441 (2022). https://doi.org/10.1007/s11571-021-09704-y
Liu, C., Zhou, C., Wang, J., et al.: Mathematical modeling for description of oscillation suppression induced by deep brain stimulation. IEEE Trans. Netural Syst. Rehabil. Eng. 26(9), 1649–1658 (2018). https://doi.org/10.1109/TNSRE.2018.2853118
Liu, F., Wang, J., Liu, C., et al.: A neural mass model of basal ganglia nuclei simulates pathological beta rhythm in Parkinson’s disease. Chaos 26(12), 123113 (2016). https://doi.org/10.1063/1.4972200
Holt, A.B., Wilson, D., Shinn, M., et al.: Phasic burst stimulation: a closed-loop approach to tuning deep brain stimulation parameters for Parkinson’s disease. PLoS Comput. Biol. 12(7), e1005011 (2016). https://doi.org/10.1371/journal.pcbi.1005011
Holt, A.B., Netoff, T.I.: Origins and suppression of oscillations in a computational model of Parkinson’s disease. J. Comput. Neurosci. 37(3), 505–521 (2014). https://doi.org/10.1007/s10827-014-0523-7
Su, F., Chen, M., Zu, L., et al.: Model-based closed-loop suppression of parkinsonian beta band oscillations through origin analysis. IEEE Trans. Netural Syst. Rehabil. Eng. 29, 450–457 (2021). https://doi.org/10.1109/TNSRE.2021.3056544
Yu, H.-T., Meng, Z.-H., Liu, C., et al.: Effective suppression of beta oscillation in Parkinsonian state via a noisy direct delayed feedback control scheme. Chin. Phys. B 30(3), 038703 (2021). https://doi.org/10.1088/1674-1056/abd395
Asadi, A., Madadi Asl, M., Vahabie, A.-H., et al.: The origin of abnormal beta oscillations in the Parkinsonian corticobasal ganglia circuits. Parkinson’s Dis. 2022, 1–13 (2022). https://doi.org/10.1155/2022/7524066
McCarthy, M.M., Moore-Kochlacs, C., Gu, X., et al.: Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 108(28), 11620–11625 (2011). https://doi.org/10.1073/pnas.1107748108
McConnell, G.C., So, R.Q., Hilliard, J.D., et al.: Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J. Neurosci. 32(45), 15657–15668 (2012). https://doi.org/10.1523/JNEUROSCI.2824-12.2012
de la Crompe, B., Aristieta, A., Leblois, A., et al.: The globus pallidus orchestrates abnormal network dynamics in a model of Parkinsonism. Nat. Commun. 11(1), 1570 (2020). https://doi.org/10.1038/s41467-020-15352-3
Xie, J., Li, T., He, T., et al.: Deep brain stimulation on the external segment of the globus pallidus improves the electrical activity of internal segment of globus pallidus in a rat model of Parkinson’s disease. Brain Res. 1797, 148115 (2022). https://doi.org/10.1016/j.brainres.2022.148115
Funding
This work was funded by the National Natural Science Foundation of China under the grant nos. 11502139, 11871377, and 12071274.
Author information
Authors and Affiliations
Contributions
Yuqin Sun finished the definition of dynamical model; Jiali Lü finished the numerical results and figures; Ye Zhou and Yingpeng Liu explained the biophysical mechanism and numerical results; Yuan Chai suggested this study and contributed to the writing of the original draft and editing of the final version. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This is a theoretical study. The Shanghai University of Electric Power Research Ethics Committee has confirmed that no ethical approval is required.
Informed consent
All authors (Yuqin Sun, Jiali Lü, Ye Zhou, Yingpeng Liu, and Yuan Chai) agree to submit and publish this work on Journal of Biological Physics.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Lü, J., Zhou, Y. et al. Suppression of beta oscillations by delayed feedback in a cortex-basal ganglia-thalamus-pedunculopontine nucleus neural loop model. J Biol Phys 49, 463–482 (2023). https://doi.org/10.1007/s10867-023-09641-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-023-09641-3