Abstract
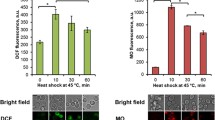
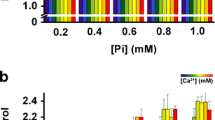
In this study we used tightly-coupled mitochondria from Yarrowia lipolytica and Dipodascus (Endomyces) magnusii yeasts, possessing a respiratory chain with the usual three points of energy conservation. High-amplitude swelling and collapse of the membrane potential were used as parameters for demonstrating induction of the mitochondrial permeability transition due to opening of a pore (mPTP). Mitochondria from Y. lipolytica, lacking a natural mitochondrial Ca2+ uptake pathway, and from D. magnusii, harboring a high-capacitive, regulated mitochondrial Ca2+ transport system (Bazhenova et al. J Biol Chem 273:4372–4377, 1998a; Bazhenova et al. Biochim Biophys Acta 1371:96–100, 1998b; Deryabina and Zvyagilskaya Biochemistry (Moscow) 65:1352–1356, 2000; Deryabina et al. J Biol Chem 276:47801–47806, 2001) were very resistant to Ca2+ overload. However, exposure of yeast mitochondria to 50–100 µM Ca2+ in the presence of the Ca2+ ionophore ETH129 induced collapse of the membrane potential, possibly due to activation of the fatty acid-dependent Ca2+/nH+-antiporter, with no classical mPTP induction. The absence of response in yeast mitochondria was not simply due to structural limitations, since large-amplitude swelling occurred in the presence of alamethicin, a hydrophobic, helical peptide, forming voltage-sensitive ion channels in lipid membranes. Ca2+- ETH129-induced activation of the Ca2+/H+-antiport system was inhibited and prevented by bovine serum albumin, and partially by inorganic phosphate and ATP. We subjected yeast mitochondria to other conditions known to induce the permeability transition in animal mitochondria, i.e., Ca2+ overload (in the presence of ETH129) combined with palmitic acid (Mironova et al. J Bioenerg Biomembr 33:319–331, 2001; Sultan and Sokolove Arch Biochem Biophys 386:37–51, 2001), SH-reagents, carboxyatractyloside (an inhibitor of the ADP/ATP translocator), depletion of intramitochondrial adenine nucleotide pools, deenergization of mitochondria, and shifting to acidic pH values in the presence of high phosphate concentrations. None of the above-mentioned substances or conditions induced a mPTP-like pore. It is thus evident that the permeability transition in yeast mitochondria is not coupled with Ca2+ uptake and is differently regulated compared to the mPTP of animal mitochondria.
Similar content being viewed by others
References
Almeida B, Buttner S, Ohlmeier S, Silva A, Mesquita A, Sampaio-Marques B, Osório NS, Kollau A, Mayer B, Leão C, Laranjinha J, Rodrigues F, Madeo F, Ludovico P (2007) J Cell Sci 120:3279–3288
Almeida B, Silva A, Mesquita A, Sampaio-Marques B, Rodrigues F, Ludovico P (2008) Biochim Biophys Acta 1783:1436–1448
Andreishcheva EN, Soares MIM, Zvyagilskaya RA (1997) Russian J Plant Physiol 44:657–664
Asimakis GK, Sordahl LA (1981) Am J Physiol 241:H671–H678
Bazhenova EN, Deryabina YI, Eriksson O, Zvyagilskaya RA, Saris N-EL (1998a) J Biol Chem 273:4372–4377
Bazhenova EN, Saris N-E, Pentilla T, Zvyagilskaya RA (1998b) Biochim Biophys Acta 1371:96–100
Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA (2006) FEBS J 273:2077–2099
Bradford MM (1976) Anal Biochem 72:248–254
Bradshaw PC, Jung DW, Pfeiffer DG (2001) J Biol Chem 276:40502–40509
Chance B, Williams GR (1955) Nature 175:1120–1121
Colin J, Garibal J, Mignotte B, Guénal I (2009) Biochem Biophys Res Commun 379:931–943
Deryabina YI, Zvyagilskaya RA (2000) Biochemistry (Moscow) 65:1352–1356
Deryabina YI, Bazhenova EN, Saris N-EL, Zvyagilskaya RA (2001) J Biol Chem 276:47801–47806
Eisenberg T, Buttner S, Kroemer G (2007) Apoptosis 12:1011–1023
Fahrenkrog B, Sauder U, Aebi U (2004) J Cell Sci 117:115–126
Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basañez G, Hardwick JM (2004) Genes Dev 18:2785–2797
Gutiérrez-Aguilar M, Pérez-Vázquez V, Bunoust O, Manon S, Rigoulet M, Uribe S (2007) Biochim Biophys Acta 1767:1245–1251
Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, Büttner S, Fehr M, Sigrist S, Madeo F (2004) J Cell Biol 164:501–507
Jamieson DJ (1998) Yeast 14:1511–1527
Jung DW, Bradshaw PC, Pfeiffer DR (1997) J Biol Chem 272:21104–21112
Kerr JF (2002) Toxicology 181–182:471–474
Kerscher S, Dröse S, Zwicker K, Zickermann V, Brandt U (2002) Biochim Biophys Acta 1555:83–91
Knorre DA, Dedukhova VI, Vyssokikh MY, Mokhova EN (2003) Biosci Rep 23(2–3):67–75
Kowaltowski AJ, Vercesi AE, Rhee SG, Netto LE (2000) FEBS Lett 473:177–180
Kristian T, Bernardi P, Siesjö BK (2001) J Neurotrauma 18:1059–1074
Laun P, Heeren G, Rinnerthaler M, Rid R, Kössler S, Koller L, Breitenbach M (2008) Biochim Biophys Acta 1783:1328–1334
Laun P, Rinnerthaler M, Bogengruber E, Heeren G, Breitenbach M (2006) Exp Gerontol 241:1208–1212
Leung AW, Halestrap AP (2008) Biochim Biophys Acta 1777:946–952
Lohret TA, Kinnally KW (1995) J Biophys 68:2299–2309
Low CP, Shui G, Liew LP, Buttner S, Madeo F, Dawes IW, Wenk MR, Yang H (2008) J Cell Sci 121:2671–2684
Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Côrte-Real M (2002) Mol Biol Cell 13:2598–2606
Lucken-Ardjomande S, Montessuit S, Martinou JC (2008) Cell Death Differ 15:923–937
Madeo F, Fröhlich KU (2008) Biochim Biophys Acta 1783:1271
Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Fröhlich KU (2002) Mol Cell 9:911–917
Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Fröhlich KU (2004) Curr Opin Microbiol 7:655–660
Manon S, Guerin M (1998) Biochim Mol Bio Int 44:565–575
Manon S, Roucou X, Guerin M, Rigoulet M, Guerin B (1998) J Bioenerg Biomembr 30:419–429
Matouschek A, Rospert S, Schmid K, Glick BS, Schatz G (1995) Proc Natl Acad Sci USA 92:6319–6323
Mironova GD, Lazareva A, Gateau-Roesch O, Tyynelä J, Zakirova R, Pavlov E, Vanier MT, Saris N-EL (1997) J Bioenerg Biomembr 26:261–269
Mironova GD, Gateau-Roesch O, Levrat C, Gritsenko E, Pavlov E, Lazareva AV, Limarenko E, Rey C, Louisot P, Saris N-EL (2001) J Bioenerg Biomembr 33:319–331
Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P (1996) J Biol Chem 271:2185–2192
Perrone GG, Tan SX, Dawes IW (2008) Biochim Biophys Acta 1783:1354–1368
Prieto S, Bouillaud F, Rial E (1995) Biochem J 307:657–661
Prieto S, Bouillaud F, Rial E (1996) Arch Biochem Biophys 334:43–49
Prieto S, Bouillaud F, Ricquier D, Rial E (1992) Eur J Biochem 208:487–491
Reiter J, Herker E, Madeo F, Schmitt MJJ (2005) Cell Biol 168:353–358
Roucou X, Manon S, Guerin M (1997) Biochim Biophys Acta 1324:120–132
Schmitt MJ, Reiter J (2008) Biochim Biophys Acta 1783:1413–1417
Severin FF, Meer MV, Smirnova EA, Knorre DA, Skulachev VP (2008) Biochim Biophys Acta 1783:1350–1353
Silva RD, Sotoca R, Johansson B, Ludovico P, Sansonetty F, Silva MT, Peinado JM, Côrte-Real M (2005) Mol Microbiol 58:824–834
Sultan A, Sokolove PM (2001) Arch Biochem Biophys 386:37–51
Wissing S, Ludovico P, Herker E, Büttner S, Engelhardt SM, Decker T, Link A, Proksch A, Rodrigues F, Corte-Real M, Fröhlich KU, Manns J, Candé C, Sigrist SJ, Kroemer G, Madeo F (2004) J Cell Biol 166(7):969–974
Zvyagilskaya RA, Selenshchikova VA, Uralskaya LA, Kotelnikova AV (1981) Biochemistry (Moscow) 46:3–10
Zvyagilskaya R, Andreishcheva E, Soares IMI, Khozin I, Berhe A, Persson BL (2001a) J Basic Microbiol 41:283–303
Zvyagilskaya R, Parchomenko O, Abramova N, Allard P, Panaretakis T, Pattison-Granberg J, Persson BL (2001b) J Membr Biol 183:39–50
Zvyagilskaya RA, Kovaleva MV, Sukhanova EI (2006) Biochim Biophys Acta Bioenergetics Short Reports 14th EBEC 14:336
Zvyagilskaya RA, Zyl’kova MV, Sukhanova EI, Kovaleva MV, Trendeleva TA (2008) Biochim Biophys Acta Bioenergetics, Short Reports 15th EBEC S32
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovaleva, M.V., Sukhanova, E.I., Trendeleva, T.A. et al. Induction of a non-specific permeability transition in mitochondria from Yarrowia lipolytica and Dipodascus (Endomyces) magnusii yeasts. J Bioenerg Biomembr 41, 239–249 (2009). https://doi.org/10.1007/s10863-009-9227-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-009-9227-5