Abstract
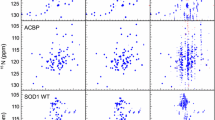
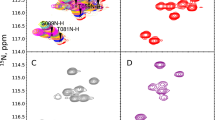
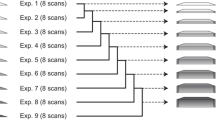
Although the order of the time steps in which the non-uniform sampling (NUS) schedule is implemented when acquiring multi-dimensional NMR spectra is of limited importance when sample conditions remain unchanged over the course of the experiment, it is shown to have major impact when samples are unstable. In the latter case, time-ordering of the NUS data points by the normalized radial length yields a reduction of sampling artifacts, regardless of the spectral reconstruction algorithm. The disadvantage of time-ordered NUS sampling is that halting the experiment prior to its completion will result in lower spectral resolution, rather than a sparser data matrix. Alternatively, digitally correcting for sample decay prior to reconstruction of randomly ordered NUS data points can mitigate reconstruction artifacts, at the cost of somewhat lower sensitivity. Application of these sampling schemes to the Alzheimer’s amyloid beta (Aβ1–42) peptide at an elevated concentration, low temperature, and 3 kbar of pressure, where approximately 75% of the peptide reverts to an NMR-invisible state during the collection of a 3D 15N-separated NOESY spectrum, highlights the improvement in artifact suppression and reveals weak medium-range NOE contacts in several regions, including the C-terminal region of the peptide.






Similar content being viewed by others
References
Ball KA et al (2011) Homogeneous and heterogeneous tertiary structure ensembles of amyloid-beta peptides. Biochemistry 50:7612–7628
Balsgart NM, Vosegaard T (2012) Fast forward maximum entropy reconstruction of sparsely sampled data. J Magn Reson 223:164–169
Barna JCJ, Laue ED, Mayger MR, Skilling J, Worrall SJP (1987) Exponential sampling, an alternative method for sampling in two-dimensional NMR experiments. J Magn Reson 73:69–77
Bermel W et al (2012) Speeding up sequence specific assignment of IDPs. J Biomol NMR 53:293–301
Billeter M (2017) Non-uniform sampling in biomolecular NMR. J Biomol NMR 68:65–66
Bostock MJ, Holland DJ, Nietlispach D (2012) Compressed sensing reconstruction of undersampled 3D NOESY spectra: application to large membrane proteins. J Biomol NMR 54:15–32
Cavini IA et al (2018) Inhibition of amyloid Ab aggregation by high pressures or specific D-enantiomeric peptides. Chem Commun (Cambridge UK) 54:3294–3297
Coggins BE, Zhou P (2008) High resolution 4-D spectroscopy with sparse concentric shell sampling and FFT-CLEAN. J Biomol NMR 42:225–239
Coggins BE, Venters RA, Zhou P (2010) Radial sampling for fast NMR: concepts and practices over three decades. Prog Nucl Magn Reson Spectrosc 57:381–419
Coggins BE, Werner-Allen JW, Yan A, Zhou P (2012) Rapid protein global fold determination using ultrasparse sampling, high-dynamic range artifact suppression, and time-shared NOESY. J Am Chem Soc 134:18619–18630
Colvin MT et al (2016) Atomic resolution structure of monomorphic A beta(42) amyloid fibrils. J Am Chem Soc 138:9663–9674
Delaglio F et al (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Delsuc MA, Tramesel D (2006) Application of maximum-entropy processing to NMR multidimensional datasets, partial sampling case. CR Chim 9:364–373
Eghbalnia HR, Bahrami A, Tonelli M, Hallenga K, Markley JL (2005) High-resolution iterative frequency identification for NMR as a general strategy for multidimensional data collection. J Am Chem Soc 127:12528–12536
Fawzi NL, Ying J, Ghirlando R, Torchia DA, Clore GM (2011) Atomic-resolution dynamics on the surface of amyloid-beta protofibrils probed by solution NMR. Nature 480:268
Hoch JC, Maciejewski MW, Mobli M, Schuyler AD, Stern AS (2014) Nonuniform sampling and maximum entropy reconstruction in multidimensional NMR. Acc Chem Res 47:708–717
Holland DJ, Bostock MJ, Gladden LF, Nietlispach D (2011) Fast multidimensional NMR spectroscopy using compressed sensing. Angew Chem Int Ed 50:6548–6551
Hou LM et al (2004) Solution NMR studies of the A beta(1–40) and A beta(1–42) peptides establish that the met35 oxidation state affects the mechanism of amyloid formation. J Am Chem Soc 126:1992–2005
Hyberts SG, Milbradt AG, Wagner AB, Arthanari H, Wagner G (2012) Application of iterative soft thresholding for fast reconstruction of NMR data non-uniformly sampled with multidimensional Poisson Gap scheduling. J Biomol NMR 52:315–327
Hyberts SG, Robson SA, Wagner G (2013) Exploring signal-to-noise ratio and sensitivity in non-uniformly sampled multi-dimensional NMR spectra. J Biomol NMR 55:167–178
Hyberts SG, Arthanari H, Robson SA, Wagner G (2014) Perspectives in magnetic resonance: NMR in the post-FFT era. J Magn Reson 241:60–73
Kamatari YO, Yokoyama S, Tachibana H, Akasaka K (2005) Pressure-jump NMR study of dissociation and association of amyloid protofibrils. J Mol Biol 349:916–921
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kazimierczuk K, Orekhov VY (2011) Accelerated NMR spectroscopy by using compressed sensing. Angew Chem Int Ed 50:5556–5559
Kern D et al (1999) Structure of a transiently phosphorylated switch in bacterial signal transduction. Nature 402:894–898
Kotler SA et al (2015) High-resolution NMR characterization of low abundance oligomers of amyloid-beta without purification. Sci Rep 5:11811
Lucast LJ, Batey RT, Doudna JA (2001) Large-scale purification of a stable form of recombinant tobacco etch virus protease. Biotechniques 30:544–544
Maciejewski MW et al (2017) NMRbox: a resource for biomolecular NMR computation. Biophys J 112:1529–1534
Mantsyzov AB et al (2014) A maximum entropy approach to the study of residue-specific backbone angle distributions in alpha-synuclein, an intrinsically disordered protein. Protein Sci 23:1275–1290
Marion D (2006) Processing of ND NMR spectra sampled in polar coordinates: a simple Fourier transform instead of a reconstruction. J Biomol NMR 36:45–54
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J Magn Reson 85:393–399
Mayzel M, Rosenlow J, Isaksson L, Orekhov VY (2014) Time-resolved multidimensional NMR with non-uniform sampling. J Biomol NMR 58:129–139
Mehlkopf AF, Korbee D, Tiggelman TA, Freeman R (1984) Sources of t1 noise in two-dimensional NMR. J Magn Reson 58:315–323
Miljenovic T, Jia XY, Lavrencic P, Kobe B, Mobli M (2017) A non-uniform sampling approach enables studies of dilute and unstable proteins. J Biomol NMR 68:119–127
Mobli M, Maciejewski MW, Gryk MR, Hoch JC (2007) Automatic maximum entropy spectral reconstruction in NMR. J Biomol NMR 39:133–139
Mobli M, Maciejewski MW, Schuyler AD, Stern AS, Hoch JC (2012) Sparse sampling methods in multidimensional NMR. Phys Chem Chem Phys 14:10835–10843
Munte CE, Erlach MB, Kremer W, Koehler J, Kalbitzer HR (2013) Distinct conformational states of the Alzheimer-amyloid peptide can be detected by high-pressure NMR spectroscopy. Angew Chem Int Ed 52:8943–8947
Orekhov VY, Jaravine VA (2011) Analysis of non-uniformly sampled spectra with multi-dimensional decomposition. Prog Nucl Magn Reson Spectrosc 59:271–292
Orekhov VY, Ibraghimov IV, Billeter M (2001) MUNIN: a new approach to multi-dimensional NMR spectra interpretation. J Biomol NMR 20:49–60
Palmer AG, Cavanagh J, Wright PE, Rance M (1991) Sensitivity improvement in proton-detected 2-dimensional heteronuclear correlation NMR-spectroscopy. J Magn Reson 93:151–170
Palmer MR et al (2015) Sensitivity of nonuniform sampling NMR. J Phys Chem B 119:6502–6515
Peterson RW, Wand AJ (2005) Self-contained high-pressure cell, apparatus, and procedure for the preparation of encapsulated proteins dissolved in low viscosity fluids for nuclear magnetic resonance spectroscopy. Rev Sci Instrum 76(9):094101
Roche J et al (2012) Cavities determine the pressure unfolding of proteins. Proc Natl Acad Sci USA 109:6945–6950
Roche J, Shen Y, Lee JH, Ying J, Bax A (2016) Monomeric A beta(1–40) and A beta(1–42) peptides in solution adopt very similar Ramachandran map distributions that closely resemble random coil. Biochemistry 55:762–775
Rovnyak D et al (2004) Accelerated acquisition of high resolution triple-resonance spectra using non-uniform sampling and maximum entropy reconstruction. J Magn Reson 170:15–21
Schanda P, Forge V, Brutscher B (2007) Protein folding and unfolding studied at atomic resolution by fast two-dimensional NMR spectroscopy. Proc Natl Acad Sci USA 104:11257–11262
Schlepckow K, Wirmer J, Bachmann A, Kiefhaber T, Schwalbe H (2008) Conserved folding pathways of alpha-lactalbumin and lysozyme revealed by kinetic CD, fluorescence, NMR, and interrupted refolding experiments. J Mol Biol 378:686–698
Schuyler AD, Hoch JC (2018) NUScon, nonuniform sampling and reconstruction challenge in NMR spectroscopy. https://nuscon.org/
Ying J, Delaglio F, Torchia DA, Bax A (2017) Sparse multidimensional iterative lineshape-enhanced (SMILE) reconstruction of both non-uniformly sampled and conventional NMR data. J Biomol NMR 68:101–118
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. We acknowledge use of the NIDDK Mass Spectrometry facility and thank Annie Geronimo and Dr. Marielle Waelti for technical support, Dr. Dennis A. Torchia, Dr. Frank Delaglio, Dr. Andy Byrd, and Dr. Hsiau-Wei Lee for valuable discussions, and Professor Vladislav Orekhov for his kind help with optimizing the MDDNMR processing scripts used to reconstruct some of the data presented in SI Figs. S3 and S6. These reconstructions were performed by making use of NMRbox: National Center for Biomolecular NMR Data Processing and Analysis, a Biomedical Technology Research Resource (BTRR), which is supported by NIH Grant P41GM111135 (NIGMS).
Funding
Funding was provided by National Institute of Diabetes and Digestive and Kidney Diseases (Grant No. DK075141-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Dennis A. Torchia on the occasion of his 80th Birthday.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ying, J., Barnes, C.A., Louis, J.M. et al. Importance of time-ordered non-uniform sampling of multi-dimensional NMR spectra of Aβ1–42 peptide under aggregating conditions. J Biomol NMR 73, 429–441 (2019). https://doi.org/10.1007/s10858-019-00235-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-019-00235-7