Abstract
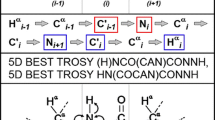
We developed an NMR pulse sequence, 3D HCA(N)CO, to correlate the chemical shifts of protein backbone 1Hα and 13Cα to those of 13C′ in the preceding residue. By applying 2H decoupling, the experiment was accomplished with high sensitivity comparable to that of HCA(CO)N. When combined with HCACO, HCAN and HCA(CO)N, the HCA(N)CO sequence allows the sequential assignment using backbone 13C′ and amide 15N chemical shifts without resort to backbone amide protons. This assignment strategy was demonstrated for 13C/15N-labeled GB1 dissolved in 2H2O. The quality of the GB1 structure determined in 2H2O was similar to that determined in H2O in spite of significantly smaller number of NOE correlations. Thus this strategy enables the determination of protein structures in 2H2O or H2O at high pH values.





Similar content being viewed by others
References
Cornilescu G, Delaglio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13:289–302
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Grzesiek S, Bax A (1992a) An efficient experiment for sequential backbone assignment of medium-sized isotopically enriched proteins. J Magn Reson 99:201–207
Grzesiek S, Bax A (1992b) Correlating backbone amide and side-chain resonances in larger proteins by multiple relayed triple resonance NMR. J Am Chem Soc 114:6291–6293
Grzesiek S, Bax A (1993) The origin and removal of artifacts in 3D HCACO spectra of proteins uniformly enriched with C-13. J Magn Reson Ser B 102:103–106
Güntert P (2004) Automated NMR structure calculation with CYANA. Methods Mol Biol 278:353–378
Ikura M, Kay LE, Bax A (1990) A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry 29:4659–4667
Juszewski K, Gronenborn AM, Clore M (1999) Improving the packing and accuracy of NMR structures with a pseudopotential for the radius of gyration. J Am Chem Soc 121:2337–2338
Kanelis V, Donaldson L, Muhandiram DR, Rotin D, Forman-Kay JD, Kay LE (2000) Sequential assignment of proline-rich regions in proteins: application to modular binding domain complexes. J Biomol NMR 16:253–259
Kay LE, Ikura M, Tschudin R, Bax A (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89:496–514
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Ottiger M, Bax A (1997) An empirical correlation between amide deuterium isotope effects on 13Cα chemical shifts and protein backbone conformation. J Am Chem Soc 119:8070–8075
Palmer AG, Cavanagh J, Wright PE, Rance M (1991) Sensitivity improvement in proton-detected two-dimensional heteronuclear correlation NMR spectroscopy. J Magn Reson 93:151–170
Wang AC, Grzesiek S, Tschudin R, Lodi PJ, Bax A (1995) Sequential backbone assignment of isotopically enriched proteins in D2O by deuterium-decoupled HA(CA)N and HA(CACO)N. J Biomol NMR 5:376–382
Wittekind M, Müller L (1993) HNCACB, a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with alpha- and beta-carbon resonances in proteins. J Magn Reson Ser B 101:201–205
Zhang W, Gmeiner WH (1996) Improved 3D gd-HCACO and gd-(H)CACO-TOCSY experiments for isotopically enriched proteins dissolved in H2O. J Biomol NMR 7:247–250
Acknowledgments
During the submission process of this manuscript, a similar pulse sequence, iH(CA)NCO, from Prof. Perttu Permi’s group was published on-line in this journal. We thank Dr. Hong-Yu Hu, Shanghai Institute of Biochemistry, Academia Sinica, China, for providing the expression vector of the Streptococcal GB1 domain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogura, K., Kumeta, H. & Inagaki, F. Structure determination of proteins in 2H2O solution aided by a deuterium-decoupled 3D HCA(N)CO experiment. J Biomol NMR 47, 243–248 (2010). https://doi.org/10.1007/s10858-010-9431-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-010-9431-y