Abstract
Methicillin resistance Staphylococcus aureus bacteria (MRSA) are serious hazards of bone implants. The present study was aimed to use the potential synergistic effects of Melittin and tetracycline to prevent MRSA associated bone implant infection. Chitosan/bioactive glass nanoparticles/tetracycline composite coatings were deposited on hydrothermally etched titanium substrate. Melittin was then coated on composite coatings by drop casting method. The surfaces were analyzed by FTIR, XRD, and SEM instruments. Tetracycline in coatings revealed multifunctional behaviors include bone regeneration and antibacterial activity. Releasing ALP enzyme from MC3T3 cells increased by tetracycline, so it is suitable candidate as osteoinductive and antibacterial agent in orthopedic implants coatings. Melittin increased the proliferation of MC3T3 cells. Composite coatings with combination of tetracycline and Melittin eradicate all MRSA bacteria, while coatings with one of them could no t eradicate all of the bacteria. In conclusion, chitosan/bioactive glass/tetracycline/Melittin coating can be suggested as a multifunctional bone implant coating because of its osteogenic and promising antibacterial activity.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, synthesis of multifunctional biomaterials have received considerable attention due to their significant potential applications [1, 2]. In this regard, many studies have been focused on designing of multifunctional nanostructures, coatings, scaffolds [3,4,5,6]. There are various combinations of bioactive and bio-inert components to improve or control the interaction of materials with their surrounding biological environment [7, 8]. In the field of orthopedics, multifunctional coatings or scaffolds are widely developed for simultaneously promoting osseointegration and antibacterial properties [9,10,11]. Aseptic loosening, infection and bacterial biofilms are in particular problems of bone implants [11,12,13]. To eradication of bacterial infection and prevent biofilm formation on the implants, antibacterial metal ions such as Ag and Cu, and antibiotics such as vancomycin and gentamycin are widely used [14,15,16,17]. Release of antibacterial metal ions caused poor proliferation and differentiation of host cells and loosening osseointegration [18]. Also long-term use of conventional antibiotics always causes bacterial resistance [19]. In this critical condition, development, design, or discovery of antimicrobial peptides (AMPs) have received much attention to dealing with antibiotic resistant bacteria and biofilm associated infections [20,21,22,23,24,25,26,27].
Melittin, an antimicrobial peptide of 26 amino acid residues, is the main component of honey bee venom with potent antibacterial activity [28,29,30]. Melittin induces pores in membranes and has a suitable synergistic effect with antibiotics on killing the resistant bacteria and, also, inhibiting biofilm formation [23, 28, 31,32,33,34].
Tetracycline a polyketide class and primarily bacteriostatic antibiotic is commonly used against a wide range of Gram-positive bacteria. Also, tetracycline is effective in the modulation of the immunoinflammatory imbalance and enhancing bone formation, decreasing connective tissue breakdown and diminishing bone resorption [35, 36]. But tetracycline is not effective against MRSA and cannot eradicate MRSA bacteria in bone implant infections [37].
Chitosan/ bioactive glass composite coatings have combined the flexibility of chitosan with strength and bioactivity of bioactive glass particles. Therefore these composites have emerged recently as new class of implant coatings with interesting properties include improved mechanical, physiochemical and biological properties [38].
Chitosan has intrinsic antibacterial activity against Staphylococcus aureus gram positive bacteria and immunomodulatory effect for accelerating angiogenesis [39, 40]. In comparison to synthetic polymers, chitosan is similar to the chemical composition of the extracellular matrix of biological systems and it can be degraded by enzymatic degradation [41]. Also, chitosan has the ability to form close-fitting films on the surface of implantable metals, ceramics and polymers which is not usually possible with other biopolymers [42]. Bioactive glass ceramics with osteogenic and bonding properties to living bone are commercially used as bone graft. Also bioactive glass matrices have been used as dental implants, bone fixation devices, and implant coatings [43]. The surface features (wettability, bioactivity, cell adhesion, and osteoconductive functioning) of the bioactive glass have been enhanced by reduction of particle size. Consequently, nanometer grain size and surface wettability of nanosized bioactive glass have led to improved selective vitronectin adsorption (a protein that mediates osteoblast adhesion), followed by modulation the promoted osteoblast adhesion and long-run functioning [43,44,45].
In the present study, we used bioactive glass nanoparticles as osteogenic agent and chitosan polymers as reservoir. According to the abovementioned issues, this study was aimed to investigate the applicability of synergism between Melittin and tetracycline as antibacterial agents in eradication of bone associated methicillin resistance Staphylococcus aurous bacteria (MRSA) infections and bone cell formation ability of coatings. Accordingly, the physiochemical, cell proliferation and differentiation and antibacterial properties of coatings were investigated.
2 Materials and methods
2.1 Coating procedure
2.1.1 Etching of titanium
Biomedical grade titanium foils (Alfa Aesar, USA) with dimensions of 5 mm × 5 mm × 0.7 mm were hydrothermally etched in H2O2 aqueous solution (6% v/v) in 170 °C for 6 h. Teflon-lined stainless steel autoclave was used for etching process and was filled up to 70%. The Ti specimens were taken out after cooling down to room temperature, ultrasonically washed by acetone, ethanol and pure water respectively and allowed to dry in ambient air.
2.1.2 Chitosan-based composite coating
Low molecular weight chitosan powders with 75–85% degree of deacetylation (Mw = 50,000–190,000 Da; Sigma-Aldrich: Germany) were dissolved at 6 g/l in 0.25% acetic acid aqueous solution. Wet synthesized bioactive glass nanoparticles with a nominal composition of (wt.%) 45 SiO2, 49 CaO, 6 P2O5 with a medium particle size of 100 nm (BGNs; Nik Ceram Razi biomedical engineering, Iran) and tetracycline (Tet; Jaber Ebn Hayyan, Iran) powders were added into the CS solution to prepare composite suspensions at proportional concentrations of antibiotic and nanoparticles. Magnetic stirring was applied for 24 h for dispersion and homogenization was done by sonication for 30 min immediately before coating. 30 µl of the solution was drop-casted over the titanium samples and then dried at ambient conditions for 24 h.
2.1.3 Peptide synthesis and coating
Melittin (GIGAVLKVLTTGLPALISWIKRKRQQ) was synthesized at DgPeptides Company (Hangzhou, China) using Fmoc chemistry. The peptide was ordered to be amidated from its C-terminal at the purity of ≥95% confirmed using Reverse-Phase HPLC by the company. Mass spectrometry was performed by the company to confirm the molecular weight of synthetic Melittin. The peptide concentration and its purity was respectively reconfirmed using bicinchoninic acid assay (BCA) and RP-HPLC as described earlier [46]. Four µl of Melittin was drop-casted over coatings and dried at ambient conditions for 3 h.
2.1.4 Experimental groups
Experimental groups and the composition of them are shown in Table 1.
2.2 Surface characterization
Scanning electron microscopy (JSM-7610F, JEOL Co., Japan and MIRA3, TESCAN Co., Czech) was used for observation of coatings’ morphology and microstructure. Surface roughness before and after hydrothermal etching was measured using a stylus instrument (MAHR POCKET SURF EMD-1500-311). The crystal structure of hydrothermally etched surface was analyzed by grazing method of X-ray diffraction diffractometer (XRD, Philips, PW1730, Cu-Kα). Fourier transform infrared spectroscopy (FTIR, ABB BomemMB100, USA) in the range of 400–4000 cm−1 with a resolution of 4 cm−1 was utilized to identify the interaction between the coatings component.
2.3 Cell culture assays
2.3.1 Cell culture
Preosteoblast MC3T3-E1 cell line was obtained from the national cell bank (Pasteur Institute of Iran, Tehran, Iran) and cultured in α-MEM supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin at 37 oC in a humidified atmosphere with 5% CO2 Proliferation medium supplemented with 1% L-ascorbic acid-2-phosphate 20 mM, 1% β- glycerophosphate 1 M and 0.00004% dexamethasone (1 mg/ml) was used for investigation of differentiation. Once confluent, the cells were collected by trypsinization of the adherent cells and resuspended in the medium. The cells were then counted using the trypan blue dye.
2.3.2 Cell proliferation assay
Cell proliferation was evaluated using the standard Alamar Blue assay protocol. Briefly, 10,000 cells were seeded on the specimens and incubated at 37 oC in 5% CO2 for 1, 3, and 7 days. After each interval, the medium discarded and new medium containing 10% of 440 mM sterile solution of resazurin in PBS was added in each well and the cells were incubated for 4 h. Absorbance was then measured at 570 nm and also 630 nm as a reference wavelength by an ELISA reader (BioTek microplate reader, USA).
2.3.3 Live/dead assay
After 2 days of cell culture, the media were discarded and specimens were washed with PBS twice. Then 200 µl of fresh medium containing 4 μM calcein AM and 10 μM ethidium homodimer-1 (Life Technologies, UK) was added to each well and incubated at 37 oC for 30 min. A fluorescence microscope (Olympus BX51, Japan) was employed for live/dead assay. Live and dead cells were respectively observed in green and red colors.
2.3.4 Alkaline phosphatase activity and DNA content assays
MC3T3 Cells were seeded onto specimens in differentiation medium. Every 2 days, the media above specimens were replaced by fresh media. Alkaline phosphatase (ALP) activity was determined on days 3, 7 and 14 after seeding of cells onto specimens. In each time point, the cells were treated by 0.5% Triton X-100 in PBS, were frozen at −80 °C, and then were thawed. Freeze-thawing was repeated three times. The solutions above specimens were assayed for ALP activity using an ALP assay kit (pNPP: Sigma-Aldrich). Enzyme activity was normalized against the total DNA content determined by Picogreen kit (Invitrogen) according to the manufacturer’s instruction.
2.4 Antibacterial assay
2.4.1 Determination of antimicrobial potential of the experimental groups
Antimicrobial activity of the specimens was tested against Methicillin-resistant Staphylococcus aureus (MRSA) bacteria strain according to our previous study [46]. Briefly, MRSA bacteria were cultured overnight. Fifty µl of bacteria solution was transferred into sterile tubes containing 2 ml of Mueller Hinton Broth (MHB) and incubated at 37 oC for 2 h to obtain bacteria in the mid-logarithmic phase of growth. Bacterial suspensions were prepared by spectrophotometry at 625 nm. According to the 0.5 McFarland standard, the optical density in the range of 0.08–0.1 is equivalent to 1.5 × 108 CFU/ml. To increase the accuracy of quantification, OD of the suspension was considered at 0.09 [47]. The numbers of bacteria was adjusted to 1.5 × 104 CFU/ml by diluting in the same medium prior to use and seeded on the specimens (three specimens per group for each time point) and incubated at 37 °C. After 6 h, the residual planktonic bacteria were plated on Mueller Hinton Agar (MHA) and incubated at 37 °C overnight and the resultant colonies were counted. For adherent bacteria, the bacterial suspension above the specimens was discarded and replaced with 200 µl PBS. The specimens were sonicated for 30 s in PBS to detach the adherent bacteria from the surfaces. The suspension was cultured on MHA as mentioned above.
2.4.2 Morphological evaluation by field emission-scanning electron microscopy
To visualize changes in cell morphology due to the effects of specimens on MRSA strain, scanning electron microscopy (SEM) was performed. The Bacteria were fixed by 2.5% glutaraldehyde in PBS and then dehydrated by 10, 30, 50, 70, 90, 100% ethanol solutions sequentially. The samples were allowed to dry at room temperature, coated with gold nanoparticles through an automatic sputter coater, and visualized using FE-SEM instruments (JSM-7610F, JEOL Co., Japan and MIRA3, TESCAN Co., Czech).
2.5 Statistical analysis
The data are presented as mean ± standard deviation (SD). The results were analyzed using ANOVA with Tukey’s post hoc t-test.
3 Results
3.1 Characterization of surfaces
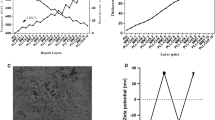
Figure 1A shows FE-SEM images of the biomedical grade Titanium sheets after hydrothermal treatment. After hydrothermal treatment, sphere shape structures were then observed. Surface roughness of the sample after hydrothermally etching was increased (Fig. 1B). Grazing incidence X-ray diffraction (GIXRD) pattern of surface after etching was shown in Fig. 1C. The reflection patterns of substrate after etching was matched with JCPDS card number 88-1175 corresponding to rutile structure. The preferable direction of TiO2 formation phase is on (200) plane 2Teta: 39.8. The preferable growth direction was determined by the most dominate reflections of the crystal planes. The morphology of coatings deposited on hydrothermally etched substrate is shown in Fig. 2.
Figure 3 shows the FTIR spectra of CS, CB, CBT, CBM and CBTM coatings and those components. The functional groups of tetracycline, bioactive glass and chitosan were revealed in composite coatings but Melittin functional groups didn’t detect in composite coatings. Melittin amount was very low, thus its functional groups didn’t reveal in FTIR spectra of CBM and CBTM coatings. The broad bonds at 3450 cm−1, 2900 cm−1, 1659 cm−1 and 1320 cm−1 are attributed to Hydroxyl group, C–H stretching, carbonyl group and amide III bond, respectively [48, 49]. The absorption bonds at 1055 and 877 cm−1 are attributed to Si-O-Si group in composite coatings [50]. Also the absorption bond at around 467 cm−1 is corresponding to PO4 [38]. Slight changes in the peak position of bioactive glass, chitosan and tetracycline were noticed in CB and CBT and CBTM composite coatings that could be attributed to hydrogen bonds formation between hydroxyl and amine groups of CS and tetracycline and oxygen of the glass network [51].
3.2 Proliferation and differentiation of MC3T3-E1 cells
The proliferation of MC3T3 cells on experimental groups was assayed by Alamar Blue test. After the first day of incubation, no significant differences in cell proliferation were observed compared to the substrate (Fig. 4). In the CS group, the cell proliferation was lower than Ti group however the difference was not significant, while the difference of cell growth in the CS group in comparison with composite coatings was significant (Fig. 4). The cells that were cultured on composite coatings proliferated more rapidly than those cultured on CS and Ti groups, after 7 days. Also, bioactive glass in composite coatings accelerated cell proliferation. Melittin and tetracycline in composite coatings have positive effect on cell proliferation compared to CS and Ti groups. Fluorescence images after live/dead staining of MC3T3 cells cultured for 2 days on experimental groups were shown in Fig. 5. The density of live MC3T3 cells on all experimental groups except for the CS coating was similar.
The secretion of ALP by MC3T3 cells that were cultured on CBTE coating was significantly higher than other groups after 5 and 10 days (Fig. 6). ALP secretion in CBG, CBME and CBTME coatings was higher than CS and Ti groups. ALP levels did not increase significantly in Ti and CS groups during 10 days (Fig. 6). These results demonstrated that composite coating causes MC3T3 to differentiate into osteoblasts and this differentiation increases when bioactive glass, tetracycline and Melittin are used as one of coating’s component.
3.3 Antibacterial performance
Antibacterial activities of the experimental groups were estimated after 6 h for both planktonic and adherent MRSA bacteria (Fig. 7A, B). CBTME coating eradicated adherent bacteria because of the synergistic effect of tetracycline and Melittin. Also, the numbers of planktonic bacteria in CBTME decreased over 3 logs compared to Ti. Reduction of both planktonic and adherent bacteria was seen in CS, CBTE and CBME coatings. SEM images of MRSA bacteria on the surfaces were shown in Fig. 8. On the CBTE, CBME and CBTME coatings, destroyed and live bacteria were seen. More bacteria were killed on CBTME coating group. Ti, CS and CB coatings were exhibited normal morphology of live MRSA bacteria without any destruction.
4 Discussion
4.1 The mechanism of coating deposition
Chitosan is a linear copolymer of glucosamine and N-acetylglucosamine that in acidic solutions, behaves as a cationic polyelectrolyte. By evaporating water from acidic solutions of chitosan, strong chitosan/ bioactive glass nanoparticles composite uniform coatings are easily formed due to the presence of hydrogen bonds between the hydroxyl and amino groups of chitosan and TiO2 layer on the etched surface and the oxygen of the bioactive glass network. FTIR results showed that tetracycline moleculs didn’t have strong bond with chitosan matrix and were released by chitosan degradation or swelling [52].
4.2 Bone regeneration capacity
In the present study we used bioactive glass nanoparticles as osteogenic agent. So, it increased ALP enzyme of MC3T3 cells and showed differentiation of them to osteoblast cells. But, the attractive result of the present research is synergism effect of tetracycline with bioactive glass on ALP secretion. Tetracycline antibiotic with chemotactic effect and anti-collagenolytic activity has guided bone regenerating potential [35]. Tetracycline with anti-inflammatory and inhibition of osteoclast function guides bone regeneration [35, 36]. Therefore, it is widely used in periodontal therapy; because of its multifunctional behavior include bone regeneration and antibacterial activity [36, 53]. Increasing ALP enzyme of MC3T3 cells by tetracycline revealed that it is a suitable candidate as osteoinductive and antibacterial agent in orthopedic implants coatings. Although, Melittin increased the proliferation of MC3T3 cells but didn’t have positive synergism on ALP production in CBME and CBTME coatings.
4.3 Antibacterial performance
The “race for the surface,” phrase points to tissue cell integration and bacterial adhesion compete for colonization on the implant’s surface [54]. Having antiadhesive or bactericidal surface for implants is necessary. Synergism of Melittin and tetracycline made antiadhesive or bactericidal surface that can eradicate adherent bacteria. So, formation of biofilm was prevented in decisive period in early stages and colonization of bone cells occurred. Killing mechanism of Melittin was destruction of bacterial membrane [31], while, tetracycline binds to bacterial ribosomes and prevents the attachment of the aminoacyl tRNA to the RNA-ribosome complex. It simultaneously inhibits other steps of the protein biosynthesis [55]. It is supposed that the penetration of tetracycline is facilitated by increasing the membrane permeability after Melittin exposure.
Chitosan and bioactive glass have antibacterial properties that influence on bactericidal properties of coatings. Amino groups of chitosan disrupt the bacterial membrane and killed them [56, 57]. Release of alkaline ions from bioactive glass increases the pH of medium. Bacteria can’t tolerate higher pH and die [58].
4.4 Clinical perspective
Antibiotic resistance is rising to dangerously high levels in all parts of the world. Antibiotic resistance leads to higher medical costs, prolonged hospital stays, and increased morbidity and mortality. The world urgently needs to change the way it prescribes and uses antibiotics. The suggestion of present study is pretreatment of orthopedic implants by combination of tetracycline and Melittin.
5 Conclusions
In the present study, titanium substrate was etched hydrothermally in H2O2 solution. Then, chitosan/bioactive glass nanoparticles/ tetracycline composite coatings were coated on etched substrate. After composite coating, Melittin as an antimicrobial peptide was drop-casted on the surface of specimens. The surfaces were assessed in vitro using both biocompatibility and antimicrobial assays. As a conclusion, tetracycline has antibacterial and osteogenic properties simultaneously. The combination of tetracycline and Melittin in the coatings decreased both planktonic and adherent MRSA bacteria, significantly. Combination of tetracycline and Melittin in the coatings eradicated adherent bacteria and prevented biofilm formation on the surface of implant.
References
Liu Z, Liu X, Ramakrishna S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol J. 2021;16:2000116 https://doi.org/10.1002/biot.202000116. p.
Chen J, Hu G, Li T, Chen Y, Gao M, Li Q, et al. Fusion peptide engineered “statically-versatile” titanium implant simultaneously enhancing anti-infection, vascularization and osseointegration. Biomaterials. 2021;264:120446 https://doi.org/10.1016/j.biomaterials.2020.120446
Ghilini F, Fagali N, Pissinis DE, Benítez G, Schilardi PL. Multifunctional titanium surfaces for orthopedic implants: antimicrobial activity and enhanced osseointegration. ACS Appl Bio Mater. 2021;4:6451–61. https://doi.org/10.1021/acsabm.1c00613
Wang B, Bian A, Jia, F, Lan J, Yang H, Yan K, et al. (2022) “Dual-functional” strontium titanate nanotubes designed based on fusion peptides simultaneously enhancing anti-infection and osseointegration. Mater Sci & Eng C. p.112650. https://doi.org/10.1016/j.msec.2022.112650
Moses, JC, Mandal BB (2022) Mesoporous silk-bioactive glass nanocomposites as drug eluting multifunctional conformal coatings for improving osseointegration and bactericidal properties of metal implants. ACS Appl Mater & Interf. https://doi.org/10.1021/acsami.2c00093
Li N, Bai J, Wang W, Liang X, Zhang W, Li W, et al. Facile and versatile surface functional polyetheretherketone with enhanced bacteriostasis and osseointegrative capability for implant application. ACS Appl Mater Inter. 2021;13:59731–46. https://doi.org/10.1021/acsami.1c19834
Gritsch L, Conoscenti G, La Carrubba V, Nooeaid P, Boccaccini AR. Polylactide-based materials science strategies to improve tissue-material interface without the use of growth factors or other biological molecules. Mater Sci Eng C. 2019;94:1083–101. https://doi.org/10.1016/j.msec.2018.09.038
Gomes BS, Simões B, Mendes PM. The increasing dynamic, functional complexity of bio-interface materials. Nat Rev Chem. 2018;2:0120 https://doi.org/10.1038/s41570-018-0120
Jia Z, Xiu P, Xiong P, Zhou W, Cheng Y, Wei S, et al. Additively manufactured macroporous titanium with silver-releasing micro-/nanoporous surface for multipurpose infection control and bone repair–a proof of concept. ACS Appl Mater Inter. 2016;8:28495–510. https://doi.org/10.1021/acsami.6b10473
Stein S, Kruck L, Warnecke D, Seitz AM, Dürselen L, Ignatius A. Osseointegration of titanium with a novel silver coating under dynamic loading. eCells Mater J. 2020;39:249–59. https://doi.org/10.22203/eCM.v039a16
Raphel J, Holodniy M, Goodman SB, Heilshorn SC. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301–14. https://doi.org/10.1016/j.biomaterials.2016.01.016
Li B, Webster TJ. Bacteria antibiotic resistance: New challenges and opportunities for implant‐associated orthopedic infections. J Orthop Res. 2018;36:22–32. https://doi.org/10.1002/jor.23656
Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections: a review. Acta Orthop. 2015;86:147–58. https://doi.org/10.3109/17453674.2014.966290
Campoccia L, Montanaro C, Arciola R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–54. https://doi.org/10.1016/j.biomaterials.2013.07.089
Alt V, Chen AF. Antimicrobial coatings for orthopaedic implants-ready for use? J Bone Jt Infect. 2020;5:125–7. https://doi.org/10.7150/jbji.46508
Fiore M, Sambri A, Zucchini R, Giannini C, Donati DM, De Paolis M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: a systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur J Orthop Sur Traumatol. 2021;31:201–20. https://doi.org/10.1007/s00590-020-02779-z
Parry MC, Laitinen MK, Albergo JI, Gaston CL, Stevenson JD, Grimer RJ, et al. Silver-coated (Agluna®) tumour prostheses can be a protective factor against infection in high risk failure patients. Eur J Surgical Oncol. 2019;45:704–10. https://doi.org/10.1016/j.ejso.2018.12.009
Song WH, Ryu HS, Hong SH. Antibacterial properties of Ag (or Pt)‐containing calcium phosphate coatings formed by micro‐arc oxidation. J Biomed Mater Res A. 2009;88:246–54. https://doi.org/10.1002/jbm.a.31877
Andersson DI. Persistence of antibiotic resistant bacteria. Curr Opin Microbiol. 2003;6:452–6. https://doi.org/10.1016/j.mib.2003.09.001
Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34:3174–83. https://doi.org/10.1016/j.biomaterials.2013.01.074
Kazemzadeh-Narbat M, Lai BF, Ding C, Kizhakkedathu JN, Hancock RE, Wang R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials. 2013;34:5969–77. https://doi.org/10.1016/j.biomaterials.2013.04.036
Pashaei F, Bevalian P, Akbari R, Bagheri KP. Single dose eradication of extensively drug resistant Acinetobacter spp. In a mouse model of burn infection by Melittin antimicrobial peptide. Microb Pathog. 2019;127:60–9. https://doi.org/10.1016/j.micpath.2018.11.055
Khozani RS, Shahbazzadeh D, Harzandi N, Feizabadi MM, Bagheri KP. Kinetics study of antimicrobial peptide, melittin, in simultaneous biofilm degradation and eradication of potent biofilm producing MDR Pseudomonas aeruginosa isolates. Int J Pept Res Ther. 2019;25:329–38. https://doi.org/10.1007/s10989-018-9675-z
Aghazadeh H, Memariani H, Ranjbar R, Pooshang Bagheri K. The activity and action mechanism of novel short selective LL‐37‐derived anticancer peptides against clinical isolates of Escherichia coli. Chem Boil Drug Des. 2019;93:75–83. https://doi.org/10.1111/cbdd.13381
Memariani H, Shahbazzadeh D, Sabatier JM, Pooshang Bagheri K. Membrane‐active peptide PV 3 efficiently eradicates multidrug‐resistant Pseudomonas aeruginosa in a mouse model of burn infection. Apmis. 2018;126:114–22. https://doi.org/10.1111/apm.12791
Memariani H, Shahbazzadeh D, Ranjbar R, Behdani M, Memariani M, Pooshang Bagheri K. Design and characterization of short hybrid antimicrobial peptides from pEM‐2, mastoparan‐VT 1, and mastoparan‐ B. Chem Boil Drug Des. 2017;89:327–38. https://doi.org/10.1111/cbdd.12864
Madanchi H, Akbari S, Shabani AA, Sardari S, Farmahini Farahani Y, Ghavami G, et al. Alignment‐based design and synthesis of new antimicrobial Aurein‐derived peptides with improved activity against Gram‐negative bacteria and evaluation of their toxicity on human cells. Drug Dev Res. 2019;80:162–70. https://doi.org/10.1002/ddr.21503
Akbari R, Vala MH, Hashemi A, Aghazadeh H, Sabatier JM, Bagheri KP. Action mechanism of Melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids. 2018;50:1231–43. https://doi.org/10.1007/s00726-018-2596-5
Dezfuli HT, Shahbazzadeh D, Eidi A, Bagheri KP, Pakravan N, Amini S, et al. Induction of IFN-γ cytokine response against hepatitis B surface antigen using melittin. Gastroenterol Hepatol Bed Bench. 2014;7:108–17.
Bevalian P, Pashaei F, Akbari R, Bagheri KP. Eradication of vancomycin-resistant Staphylococcus aureus on a mouse model of third-degree burn infection by melittin: an antimicrobial peptide from bee venom. Toxicon. 2021;199:49–59. https://doi.org/10.1016/j.toxicon.2021.05.015
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50. https://doi.org/10.1038/nrmicro1098
Bardbari AM, Arabestani MR, Karami M, Keramat F, Aghazadeh H, Alikhani MY, et al. Highly synergistic activity of Melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2018;37:443–54. https://doi.org/10.1007/s10096-018-3189-7
Akbari R, Hakemi-Vala M, Pashaie F, Bevalian P, Hashemi A, Pooshang Bagheri K. Highly synergistic effects of Melittin with conventional antibiotics against multidrug resistant strains of Acinetobacter baumannii and Pseudomonas aeruginosa. Micro Drug Resist. 2019;25:193–202. https://doi.org/10.1089/mdr.2018.0016
Zarghami V, Ghorbani M, Bagheri KP, Shokrgozar MA. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater Chem Phys. 2021;263:124432 https://doi.org/10.1016/j.matchemphys.2021.124432
Park YJ, Lee YM, Park SN, Lee JY, Ku Y, Chung CP, et al. Enhanced guided bone regeneration by controlled tetracycline release from poly (L‐lactide) barrier membranes. J Biomed Mater Res A. 2000;51:391–7. https://doi.org/10.1002/1097-4636(20000905)51:3<391::AID-JBM13>3.0.CO;2-9
Gomes PS, Santos JD, Fernandes MH. Cell-induced response by tetracyclines on human bone marrow colonized hydroxyapatite and Bonelike. Acta Biomater. 2008;4:630–7. https://doi.org/10.1016/j.actbio.2007.12.006
Trzcinski K, Cooper BS, Hryniewicz W, Dowson CG. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2000;45:763–70. https://doi.org/10.1093/jac/45.6.763
Boccaccini AR, Erol M, Stark WJ, Mohn D, Hong Z, Mano JF. Polymer/bioactive glass nanocomposites for biomedical applications: a review. Compos Sci Technol. 2010;70:1764–76. https://doi.org/10.1016/j.compscitech.2010.06.002
Zheng LY, Zhu JF (20031) Study on antimicrobial activity of chitosan with different molecular weights, Carbohydr Polym. 54: 527-30 https://doi.org/10.1016/j.carbpol.2003.07.009
Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. https://doi.org/10.1016/j.biomaterials.2005.03.016
Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. https://doi.org/10.1016/j.addr.2009.09.004
LogithKumar R, KeshavNarayan A, Dhivya S, Chawla A, Saravanan S, Selvamurugan NA. Review of chitosan and its derivatives in bone tissue engineering. Carbohydr Polym. 2016;151:172–88. https://doi.org/10.1016/j.carbpol.2016.05.049
Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457–86. https://doi.org/10.1016/j.actbio.2012.08.023
Baino F, Verné E. Glass-based coatings on biomedical implants: a state-of-the-art review. Biomed Glasses. 2017;3:1–17. https://doi.org/10.1515/bglass-2017-0001
Sergi R, Bellucci D, Cannillo V. A comprehensive review of bioactive glass coatings: state of the art, challenges and future perspectives. Coatings. 2020;10:757–85. https://doi.org/10.3390/coatings10080757
Zarghami V, Ghorbani M, Bagheri KP, Shokrgozar MA. Prolongation of bactericidal efficiency of chitosan—Bioactive glass coating by drug controlled release. Prog Org Coat. 2020;139:105440 https://doi.org/10.1016/j.porgcoat.2019
Eisapoor SS, Jamili S, Shahbazzadeh D, Ghavam Mostafavi P, Pooshang Bagheri K. A new, high yield, rapid, and cost-effective protocol to deprotection of cysteine-rich conopeptide, omega-conotoxin MVIIA. Chem Biol Drug Des. 2016;87:687–93. https://doi.org/10.1111/cbdd.12702. 105440
Yang CC, Lin C, Yen SK. Electrochemical deposition of vancomycin/chitosan composite on Ti alloy. J Electrochem Soc. 2011;158:E152–8. https://doi.org/10.1149/2.105112jes
Gebhardt F, Seuss S, Turhan MC, Hornberger H, Virtanen S, Boccaccini AR. Characterization of electrophoretic chitosan coatings on stainless steel. Mater Lett. 2013;66:302–4. https://doi.org/10.1016/j.matlet.2011.08.088
Mehdipour M, Afshar A, Mohebali M. Electrophoretic deposition of bioactive glass coating on 316L stainless steel and electrochemical behavior study. Appl Surf Sci. 2012;258:9832–9. https://doi.org/10.1016/j.apsusc.2012.06.038
Ordikhani F, Simchi A. Long-term antibiotic delivery by chitosan-based composite coatings with bone regenerative potential. Appl Surf Sci. 2014;317:56–66. https://doi.org/10.1016/j.apsusc.2014.07.197
Cai X, Ma K, Zhou Y, Jiang T, Wang Y. Surface functionalization of titanium with tetracycline loaded chitosan–gelatin nanosphere coatings via EPD: fabrication, characterization and mechanism. RSC Adv. 2016;6:7674–82. https://doi.org/10.1039/C5RA17109A
Chung CP, Ki DK, Park YJ, Nam KH, Lee SJ. Biological effects of drug‐loaded biodegradable membranes for guided bone regeneration. J Periodontal Res. 1997;32:172–5. https://doi.org/10.1111/j.1600-0765.1997.tb01400.x
Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–95. https://doi.org/10.1126/science.3629258
Hancock REW. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis. 2005;5:209–18. https://doi.org/10.1016/S1473-3099(05)70051-7
Bae K, Jun EJ, Lee SM, Paik DI, Kim JB. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Invest. 2006;10:102 https://doi.org/10.1007/s00784-006-0038-3
Xie W, Xu P, Wang W, Liu Q. Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohydr Polym. 2002;50:35–40. https://doi.org/10.1016/S0144-8617(01)00370-8
Echezarreta-López MM, Landin M. Using machine learning for improving knowledge on antibacterial effect of bioactive glass. Int J Pharm. 2013;453:641–7. https://doi.org/10.1016/j.ijpharm.2013.06.036
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarghami, V., Ghorbani, M., Bagheri, K.P. et al. Improving bactericidal performance of implant composite coatings by synergism between Melittin and tetracycline. J Mater Sci: Mater Med 33, 46 (2022). https://doi.org/10.1007/s10856-022-06666-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-022-06666-3