Abstract
We reported Langmuir–Blodgett films of molybdenum disulphide and tungsten disulfide and studied the formation of \({\text{Mo}}{\text{S}}_{2}\) and \({\text{WS}}_{2}\) layers. The effect of the subphase pH is investigated. Also, the influence of the subphase conditions on the number of layers was reported. By varying the pH of the buffer solution subphase, the solvent-to-nanoparticle interaction is changed and the compression of the layer during deposition is influenced. The stacking of the \({\text{Mo}}{\text{S}}_{2}\) and \({\text{WS}}_{2}\) crystalline structure under the influence of subphase pH and the material concentration were investigated. Langmuir layers assembly is a useful method to manipulate the distribution of \({\text{WS}}_{2}\) and \({\text{Mo}}{\text{S}}_{2}\). Successful deposition of dichalcogenides at the air–water interface on silicon substrate was performed using Balance Langmuir–Blodgett technique. The characterization of \({\text{WS}}_{2}\) and films \({\text{Mo}}{\text{S}}_{2}\) with X-ray diffraction, Raman spectroscopy, atomic force microscopy and scanning electron microscopy was carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The small world of matter is getting smaller and smaller. In recent years, nanoscience was greatly developed that have allowed nanotechnologists to take giant steps in materials science and technology. Numerous applications in a wide range of fields are very beneficial to humans, and much research in development is very promising. Applications in medicine [1,2,3], industry [4,5,6,7], electronics [8, 9], telecommunications [10, 11], energy [12,13,14] or aeronautics [15] are just some examples of areas where there are enormous benefits, and the potential is clear. Some nanotechnologies are already being applied, and others are under development or test phase [16].
Two-dimensional (2D) materials exhibit a plethora of novel properties, including structural [17], optical [18], and electronic characteristics [19, 20], which have garnered significant attention and research efforts over the past decades, owing to their diverse range of applications [21]. The most widely studied 2D material is graphene, because of its rich physical properties and high carrier mobility. However, pristine graphene does not have a bandgap, a property that is essential for many optoelectronic applications including transistors. Engineering a graphene bandgap increases fabrication complexity and either reduces mobilities to the level of strained silicon films or requires high voltage. Although 2D-layered transition metal dichalcogenides (2DTMDs), like single layers of Molybdenum disulphide (\({\text{MoS}}_{2}\)) and Tungsten disulphide (\({\text{WS}}_{2}\)), have a relatively large intrinsic direct bandgaps, 1.8 and 2.05 eV, respectively, previously reported mobilities in the \(0.5-3{\text{ cm}}^{2}/\text{Vs}\) range are too low for practical devices [22]. However, the room temperature mobilities of \(\sim 200 {\text{ cm}}^{2}/\text{Vs}\) were reported in \({\text{MoS}}_{2}\) single layer [23], like that of graphene ribbons [24].
The deposition method via using the Langmuir–Blodgett balance, apart from being used for the manufacture of Langmuir thin films, can be also applied to build highly organized multi-layer structures of amphiphilic molecules. This is achieved by successive immersion and emersion of a solid substrate. Across the water/air interface, the transfer of the nanomaterial is maintained at a constant surface pressure [25], which is controlled by a feedback system between the tensiometer and the barrier control mechanism. In this way, the floating monolayer is absorbed again and again onto the solid substrate [26].
2 Materials and methods
2.1 The Materials
Two kinds of 2D materials were used, \(Mo{S}_{2}\) and \({\text{WS}}_{2}\). The dichalcogenides were obtained from 2D semiconductors with a concentration of 0.01 mg/ml isopropanol solution to form a colloidal dispersion, which was used as spreading solution. \(Mo{S}_{2}\) and \({\text{WS}}_{2}\) layers were fabricated using Langmuir–Blodgett trough (KSV Nima LB Trough Medium KN 103). Ultra-filtered and de-ionized water (Millipore 18.2 MΩ) was used as subphase. Subphase used for monolayer formation was an aqueous solution of 10 mM sodium-calcium buffer saline \(\left(\text{NaCl}-{\text{CaCl}}_{2}\right)\) and 5 mM calcium buffer saline. 5 mM calcium buffer saline \(\left({\text{Na}}_{2}{\text{Co}}_{3}\right)\) buffered to pH 7.2 and 5.0, respectively. Sodium chloride (> 99.99% purity) \(\text{NaCl}\), calcium chloride (> 96% purity) (\({\text{CaCl}}_{2}\)), sodium nitrate (> 99% purity) were purchased from Sigma-Aldrich and the silicon wafers were purchased from Siltronix.
2.2 Preparation of LB WS2 and MoS2 films on Si/Si O2
Si/Si \({O}_{2}\) was cleaned in sulfochromic acid \(({H}_{2}Cr{O}_{4})\), for 60 s, then rinsed with DI water and ethanol and dried with the help of 99% pure \({N}_{2}\) flow. The trough was cleaned with chloroform \((CHC{l}_{3})\), followed by DI, and was dried using nitrogen gas to obtain a contamination-frees subphase for films.
The film formation was resulted from de following procedure. Before the deposition, the material is ultrasonically treated for 5 min to obtain stable \({\text{WS}}_{2}\) and \({\text{Mo}}{\text{S}}_{2}\) solution. The solution of material was dipped into a trough containing buffer solution and spread on the surface of the cell. The trough contained 80 mL of buffer solution with pH of 5 and 7.4. For the LB deposition experiments, 0.8 mL and 1 mL of material solution was spread using a Hamilton microsyringe on the subphase and allowed to stabilize for 12 h before compression. Then, the cleaned substrate was clamped to the dipper, and barriers were compressed at a speed of 1 mm/min to dense material layer on the water surface. The LB films were deposited on substrates using the vertical dipping at a rate of 1 mm/min. The depositions were made at a constant pressure of 13 mN/m, and the surface pressure versus area per molecule (\(\pi -A\)) is monitored by a sensor (Wilhelmy plate). Finally, a \(Mo{S}_{2}\) film was annealed in a drying oven at a temperature of 180 °C. After 4 h, a \(Mo{S}_{2}\) film coated on the surface of substrate was obtained.
3 Results and discussion
The results obtained in this work are related to 4 samples listed in Table 1, where the pH and the buffer solution are the unique parameter variety in the LB deposition.
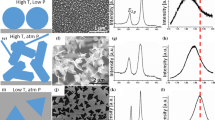
The material films on silicon were observed with a scanning electron microscope (model JSM-7401F). Scanning electron (SEM) micrographs obtained are shown in Fig. 1. In Fig. 1a the film deposited on silicon from sample A, and Fig. 1b is the 1a zoom. The film of sample B is shown in Fig. 1c, and Fig. 1d is the 1c zoom. In short, it was determined that the surface of \(Mo{S}_{2}\) films was very uniform for sample B. Moreover, we could estimate the particle size in the \(Mo{S}_{2}\) films, and it was about 1 and 5 μm for samples A and B, respectively.
Figure 1e shows the film deposited on silicon from sample C, and Fig. 1f is Fig. 1e zoom. In Fig. 1g is the film of sample D, and Fig. 1h is Fig. 2g zoom. In the SEM images, one can see that the \(W{S}_{2}\) distribution in large scale is not uniform. Therefore, when we adjust the pH of the buffer solution in the sample D, we can observe the crystal structure of triangles of \(W{S}_{2}\), as it is shown in Fig. 1h.
The transfer behavior of films on \(\text{Si}/{\text{SiO}}_{2}\) substrates was studied. The results reached for samples A and B are shown in Fig. 2. The AFM image presented in Fig. 2a shows the 3D topographic surface and roughness of the sample A, and one can observe staking of \({\text{MoS}}_{2}\) triangles. In contrast, the uniform \({\text{MoS}}_{2}\) film of sample B is observed in Fig. 2c. These features are clearly seen in a typical histogram of height distribution across a film. In this case, the height zero is defined as the maximum depth that AFM tip can probe, as shown in the histograms in Fig. 2b and Fig. 2d to samples A and B, respectively. Here, one can observe that for sample A, more than 90% of nanotriangles were average heights ranging from 300 to 500 A° and the surface rms roughness, ≈110 A°, while for sample B, the height profile (inset of Fig. 2d) showed that the thin film was averaged heights ranging from 79 to 81 A° and the surface rms roughness, ≈6.44 A°, which was significantly lower than the previous sample.
As to AFM characterization of \({\text{WS}}_{2}\), in Fig. 2e–g are the roughness by 3D topographic surface and the related height distributions are shown in Fig. 2f–h. As seen in Fig. 2e, the film is composed of small triangles in the sample C, and for the sample D, one can observe greater homogeneity (Fig. 2f).
The bearing ratio, on the other hand, goes to zero beyond 100 and 650 A˚ for films A and B, respectively, and beyond 140 and 1500 A˚ for films C and D, indicating that the structures are connected to each other (at least for the resolution dictated by the scan size and the aspect ratio of the tip) at this depth from the surface. This is true as the scan size was much larger than the size of the island. One can also obtain the average shape of these islands as a function of depth from the bearing ratio distribution.
For further characterization of the \({\text{WS}}_{2}\) and \({\text{Mo}}{\text{S}}_{2}\) thin films, Raman spectroscopy was applied to verify the exfoliation of bulk materials into few-layer nanosheets. The Raman spectra of \({\text{Mo}}{\text{S}}_{2}\) thin films for the sample A and B and their bulk as a reference are represented in Fig. 3a. The insert Fig. 3a is a zoom in the peaks of \(Mo{S}_{2}\) compared to Raman spectrum of bulk material. The characteristic modes \({E}_{2g}^{1}(\Gamma )\) (in-plane vibration of Mo and S atoms) and \({A}_{1g }(\Gamma )\) (out-of-plane vibration of S atoms) of hexagonal phase \(Mo{S}_{2}\) were observed at around 383 \(c{m}^{-1}\) and 409 \(c{m}^{-1}\) with a difference of 26 \(c{m}^{-1}\) Raman shift (frequency difference, D = \({{A}_{1g }\left(\Gamma \right)-E}_{2g}^{1}(\Gamma )\)) which is consistent with the typical value of bulk \(Mo{S}_{2}\), respectively.
For further characterization of the \(W{S}_{2}\) thin films, Raman spectroscopy was applied to verify the deposition of monolayer (Fig. 3b sample D), few layers (Fig. 3b sample C) and bulk (Fig. 3c). In Fig. 3b there are typical Raman spectra of monolayer \({\text{WS}}_{2}\) regions. For \({\uplambda }_{\text{exc}}=514.4\text{ nm}\), the Raman spectrum is dominated by the first-order optical modes: \({E}_{2g}^{1}\left(\Gamma \right)\) at 350 and \({A}_{1g }\left(\Gamma \right)\) at 418 \({\text{cm}}^{-1}\) modes LA(M) at 176 \({\text{cm}}^{-1}\). The resonant Raman spectrum involves the longitudinal acoustic phonons at the M point of the Brillouin zone \(\left[\text{LA}\left(\text{M}\right)\text{at }176\text{ c}{\text{m}}^{-1}\right]\), overtones \([2\text{LA}\left(\text{M}\right)\text{at }297\text{ c}{\text{m}}^{-1}\text{ and}\) \(4\text{LA}\left(\text{M}\right)\text{ at }700\text{ c}{\text{m}}^{-1}]\) second and fourth harmonics and combination modes \({\text{A}}_{1\text{g }}(\text{M})\) at 230 \({\text{cm}}^{-1}\), 2LA(M) at 324 \({\text{cm}}^{-1}.\) The samples contain a large number of monolayer islands that is suitable to study the thickness dependence of the Raman response. The absolute intensity of the 2LA(M) node increases with decreasing the number of layers, while the intensity of the \({\text{A}}_{1\text{g }}(\Gamma )\) displays the opposite trend, and it presumably results from weaker interlayer contribution to the phonon restoring forces [21].
X-ray diffraction spectra (XRD) for the films on silicon were recorded to obtain the structure and phase composition of the materials. In Fig. 4a there is a diffraction pattern of the film of the sample B, as fabricated using pH 7.4. Here, the resulting film is seen to be highly oriented, and its crystallites are deposited in such state that all their basal planes are parallel to the substrate. The film exhibited two diffraction peaks at 2θ = 32.82° and 2θ = 58.78° corresponding to (100) and (110) planes of hexagonal \({\text{MoS}}_{2}\). Another observed peak at 2θ = 61.97° was attributed to the (008) plane of hexagonal \({\text{MoS}}_{2}\), respectively (JPDS:37–1492) [27]. The peak 2θ = 70° is corresponding to the (400) crystal plane of silicon [28, 29]. In Fig. 4b there is the WRD pattern of \({\text{WS}}_{2}\) (sample D). All the square marked peaks match well with standard hexagonal \({\text{WS}}_{2}\) diffraction peaks (JCPS standard card no. 84–1398) [30, 31]. From Fig. 4b, the XRD pattern characteristic peaks at 30.2°, 32.40°, 35.62°, 54.59°, 56.32° and 62.01° are corresponding to crystal planes (100), (101), (103), (006), (110) and (112) [32, 33]. In planes 100 and 110 which is in accordance with the plane 2H-W S2 crystal, thus confirming hexagonal crystal system of \({\text{WS}}_{2}\) [34, 35]
4 Conclusion
High coverage and high-quality 2D colloidal crystals were successfully fabricated on Si wafers, and the study of the pH effect on \({\text{WS}}_{2}\) and \({\text{MoS}}_{2}\) thin films using different buffer solutions by Langmuir–Blodgett Balance is presented. In the first part, the thins films were characterized using various spectroscopic and microscopic techniques (Raman spectroscopy, SEM, and AFM). LBB deposition method provide a strong confinement, but pH is a parameter related with the crystalline structure and numbers of layer of the film. We have demonstrated the obtaining of monolayer films with length of around 5 µm via the Langmuir–Blodgett balance using pH value of 7.2, a short analysis by varying pH of the buffer solution to deposit the materials was performed. The investigation of the deposition of 2D materials free chemical residues is an imperative for fabrication of semiconductor FET devices, and monolayer of material with indirect bandgap may be useful for improving the accuracy and response of photodetectors.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
Code availability
Not applicable.
References
M.S. Bagherian, P. Zargham, H. Zarharan, M. Bakhtiari, N. Mortezaee Ghariyeh Ali, E. Yousefi, A. Es-Haghi, M.E. Taghavizadeh Yazdi, Antimi-crobial and antibiofilm properties of selenium-chitosan-loaded salicylic acid nanoparticles for the removal of emerging contaminants from bacterial pathogens. World J. Microbiol. Biotechnol. 40(3), 86 (2024)
R.J. Wilson, Y. Li, G. Yang, C.X. Zhao, Nanoemulsions for drug delivery. Particuology 64, 85–97 (2022)
B. Özuğur Uysal, Ş Nayır, M. Açba, B. Çıtır, S. Durmaz, Ş Koçoğlu, E. Yıldız, Ö. Pekcan, 2d materials (ws2, mos2, mose2) enhanced polyacrylamide gels for multifunctional applications. Gels 8(8), 465 (2022)
H. Pérez, O.J. Quintero García, M.A. Amezcua-Allieri, R. Rodriguez Vazquez, Nanotechnology as an efficient and effective alternative for wastewater treatment: an overview. Water Sci. Technol. 87(12), 2971–3001 (2023)
X. Fan, X. Li, Z. Zhao, Z. Yue, P. Feng, X. Ma, H. Li, X. Ye, M. Zhu, Het- erostructured rgo/mos2 nanocomposites toward enhancing lubrication function of industrial gear oils. Carbon 191, 84–97 (2022)
M.A. Shah, B.M. Pirzada, G. Price, A.L. Shibiru, A. Qurashi, Applications of nanotechnology in smart textile industry: a critical review. J. Adv. Res. 38, 55–75 (2022)
Z.H. Mohammad, F. Ahmad, S.A. Ibrahim, S. Zaidi, Application of nan- otechnology in different aspects of the food industry. Discov. Food 2(1), 12 (2022)
Z. Wang, Y. Yang, B. Hua, Q. Ji, Synthetic two-dimensional electronics for transistor scaling. Front. Phys. 18(6), 63601 (2023)
T.K. Sahu, N. Kumar, S. Chahal, R. Jana, S. Paul, M. Mukherjee, A.H. Tavabi, A. Datta, R.E. Dunin-Borkowski, I. Valov et al., Microwave synthesis of molybdenene from mos2. Nat. Nanotechnol. 18(12), 1430–1438 (2023)
Y. Yu, S. Liu, C.-M. Lee, P. Michler, S. Reitzenstein, K. Srinivasan, E. Waks, J. Liu, Telecom-band quantum dot technologies for long distance quantum networks. Nat. Nanotechnol. 18(12), 1389–1400 (2023)
C. Hong, S. Oh, V.K. Dat, S. Pak, S. Cha, K.H. Ko, G.M. Choi, T. Low, S.H. Oh, J.H. Kim, Engineering electrode interfaces for telecom-band photodetection in MoS2/Au heterostructures via sub-band light absorption. Light Sci. Appl. 12(1), 280 (2023)
C.T. Chen, Y. Fu, X. Gao, A. Butler, K. Reyes, H. Li, M. Pentaris, A. Yadav, K.T. Wong, H. Yue et al., Two-dimensional van der waals materials and their mixed low-dimensional hybrids for electrochemical energy applications. MRS Bull. 48(9), 932–946 (2023)
Z. Zhu, G. Xiao, J. Chen, S. Fu, Wood nanotechnology: a more promising solution toward energy issues: a mini-review. Cellulose 27(8513), 8526 (2020)
D. Łukawski, P. Hochmańska-Kaniewska, D. Janiszewska-Latterini, A. Lekawa-Raus, Functional materials based on wood, carbon nanotubes, and graphene: manufacturing, applications, and green perspectives. Wood Sci. Technol. 57(5), 989–1037 (2023)
E. Cestino, J. Catapano, F. Galvano, A. Felis, S. Zuccalà, V. Martilla, V. Sapienza, L. Chesta, Effectiveness of nanotechnology treatments in composite aircraft applications. Appl. Sci. 14(5), 1721 (2024)
Filipe JA, Ferreira MA. (2021). Analysis of nanosciences and nanotechnology and their applications. arXiv preprint arXiv:2111.03425.
Y. Huang, J. Guo, Y. Kang, Y. Ai, C.M. Li, Two dimensional atomically thin mos 2 nanosheets and their sensing applications. Nanoscale 7(46), 19358–19376 (2015)
H.R. Gutiérrez, N. Perea-López, A.L. Elías, A. Berkdemir, B. Wang, R. Lv, F. López-Urías, V.H. Crespi, H. Terrones, M. Terrones, Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 13(8), 3447–3454 (2013)
A. Sebastian, R. Pendurthi, T.H. Choudhury, J.M. Redwing, S. Das, Benchmarking monolayer mos2 and ws2 field-effect transistors. Nat. Commun. 12(1), 693 (2021)
B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti, A. Kis, Single-layer MoS2 transistors. Nat. Nanotechnol. 6(3), 147–150 (2011)
W. Cao, J. Kang, D. Sarkar, W. Liu, K. Banerjee, 2d semiconductor fets—projections and design for sub-10 nm vlsi. IEEE Trans. Electron Devices 62(1), 3459–3469 (2015)
Z. Yu, Z.Y. Ong, S. Li, J.B. Xu, G. Zhang, Y.W. Zhang, Y. Shi, X. Wang, Analyzing the carrier mobility in transition-metal dichalcogenide mos2 field-effect transistors. Adv. Funct. Mater. 27(19), 1604093 (2017)
Alzakia, F.I. (2021). Liquid exfoliation of two-dimensional materials for optoelectronic device applications. PhD thesis, National University of Singapore (Singapore)
I. Langmuir, The constitution and fundamental properties of solids and liquids. ii. liquids. J. American chem. soci. 39(9), 1848–1906 (1917)
Biolin Scientific: Langmuir-Blodgett Trough - Manual. Technical report, KSV NIMA, Finland (2013)
Q. Fu, B. Xiang, Monolayer transition metal disulfide: synthesis, characterization and applications. Prog. Nat. Sci.: Mater. Int. 26(3), 221–231 (2016)
Y. Zhang, Y. Li, H. Li, F. Yin, Y. Zhao, Z. Bakenov, Synthesis of hierarchical mos 2 microspheres composed of nanosheets assembled via facile hydrothermal method as anode material for lithium-ion batteries. J. Nanopart. Res. 18, 1–9 (2016)
N. Akcay, M. Tivanov, S. Ozcelik, Mos 2 thin films grown by sulfurization of dc sputtered mo thin films on si/sio 2 and c-plane sapphire substrates. J. Electron. Mater. 50, 1452–1466 (2021)
A. Kuc, N. Zibouche, T. Heine, Influence of quantum confinement on the electronic structure of the transition metal sulfide t s 2. Phys. Rev. B 83(24), 245213 (2011)
S. Qi, X. Liu, N. Ma, H. Xu, Construction and photocatalytic properties of ws2/mos2/biocl heterojunction. Chem. Phys. Lett. 763, 138203 (2021)
X. Li, J. Zhang, Z. Liu, C. Fu, C. Niu, Ws2 nanoflowers on carbon nanotube vines with enhanced electrochemical performances for lithium and sodium-ion batteries. J. Alloy. Compd. 766, 656–662 (2018)
S. Manjunatha, T. Machappa, A. Sunilkumar, Y. Ravikiran, Tungsten disulfide: an efficient material in enhancement of ac conductivity and dielectric properties of polyaniline. J. Mater. Sci. Mater. Electron. 29, 11581–11590 (2018)
M. Genut, L. Margulis, G. Hodes, R. Tenne, Preparation and microstructure ws2 thin films. Thin Solid Films 217(1–2), 91–97 (1992)
Y. Chen, L. Gan, H. Li, Y. Ma, T. Zhai, Achieving uniform monolayer transition metal dichalcogenides film on silicon wafer via silanization treatment: a typical study on ws2. Adv. Mater. 29(7), 1603550 (2017)
I. Hotovy, L. Spiess, M. Mikolasek, I. Kostic, H. Romanus, Structural and morphological evaluation of layered ws2 thin films. Vacuum 179, 109570 (2020)
Acknowledgements
Authors are obliged and gratified to the Lab de caracterizacion de materiales por AFM y Micro-Raman, Dr. Rogelio J. Fragoso Soriano, MSc. Ana Bertha Soto Guzmán and Marcela Guerrero Cruz.
Funding
This work was supported by the Consejo Nacional de Humanidades Ciencias y Tecnologias (CONAHCYT) and Centro de investigacion y de Estudios Avanzados (CINVESTAV).
Author information
Authors and Affiliations
Contributions
All authros contributed to the study, conception and design of this experimental work. Material preparation, characterization and their analysis were performed by AARH, PCM and SK. The first draft of the manuscript is written by AARH and proof read by MGR and DVP. At last all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Ethical approval for this type of study is formal consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosales Hernández, A.A., Córdova Morales, P., Gujari, S.K. et al. pH effect in Langmuir–Blodgett self-assembly of MoS2 and WS2 thin films. J Mater Sci: Mater Electron 35, 1326 (2024). https://doi.org/10.1007/s10854-024-12994-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12994-7