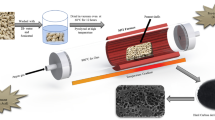
Abstract
In this study, NiO–SiO2-based composites were synthesized through low-temperature aqueous chemical growth utilizing a facile, low-cost, and environmentally friendly approach. The composite systems were prepared using a combination of silica gel and citrus lemon peel extract. Due to the remarkable green chemicals in orange peel extract, porous nanostructures have been developed with thin sheet-like properties. The composite materials were examined in terms of their crystalline structure, morphology, optical band gap, and surface chemical composition. An advanced non-enzymatic glucose sensor developed from NiO–SiO2 composites exhibits rich surface oxygen vacancies and abundant catalytic sites. Based on sample 2, cyclic voltammetry revealed a linear glucose concentration range between 0.1 and 20 mM, chronoamperometry exhibited glucose concentration ranges between 0.1 and 14 mM, and linear sweep voltammetry revealed glucose concentration ranges from 0.1 to 10 mM. In enzymatic glucose sensors, the minimum level of detection was estimated to be 0.08 mM. A number of sensor characterization parameters were examined, including selectivity, stability, reproducibility, and real-time applications. In addition, electrochemical impedance spectroscopy (EIS) has shown that the NiO–SiO2 composite performs well in non-enzymatic glucose sensing due to its low charge transfer resistance and high electrochemical active surface area (ECSA). NiO–SiO2 composites could have significant biomedical, energy conversion, and storage applications based on the results obtained.









Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
K. Justice Babu, S. Sheet, Y.S. Lee, G. Gnana Kumar, Three-dimensional dendrite Cu–Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection. ACS Sustain. Chem. Eng. 6, 1909–1918 (2018)
X. Qian, A. Ko, H. Li, C. Liao, Flexible non-enzymatic glucose strip for direct non-invasive diabetic management. Microchem. J. 197, 109818 (2024)
S. Zhang, W. Zhao, C. Liu, J. Zeng, Z. He, C. Wang, W. Yuan, Q. Wang, Flower-like CoO nanowire-decorated Ni foam: a non-invasive electrochemical biosensor for glucose detection in human saliva. Appl. Mater. Today 36, 102083 (2024)
W.-W. Zhao, J.-J. Xu, H.-Y. Chen, Photoelectrochemical enzymatic biosensors. Biosen. Bioelectron. 92, 294–304 (2017)
Z. Wang, H. Lei, L. Feng, A facile channel for D-glucose detection in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 114, 293–297 (2013)
C. Guati, L. Gomez-Coma, M. Fallanza, I. Ortiz, Progress on the influence of non-enzymatic electrodes characteristics on the response to glucose detection: a review (2016–2022). Rev. Chem. Eng. 40, 123–148 (2024)
D. Arif, M. Hassan, M. Abdullah, W. Miran, M.A. Nasir, S. Batool, M.A. Baig, and U. Liaqat, An electrochemical sensor based on copper oxide nanoparticles loaded on a mesoporous MCM-41 for non-enzymatic detection of glucose. Ceramics International. (2024).
V. Subramanian, A. Humayun, P. Nagomony, C. Viswanathan, Non-enzymatic electrochemical detection of methylglyoxal in saliva using a polyaniline/nickel oxide nanohybrid biosensor: A noninvasive approach for diabetes diagnosis. Biosens. Bioelectron. X, 100444 (2024)
R. Chen, W. Xu, C. Xiong, X. Zhou, S. Xiong, Z. Nie, High-salt-tolerance matrix for facile detection of glucose in rat brain microdialysates by MALDI mass spectrometry. Anal. chem. 84, 465–469 (2012)
S. Majeed, W. Gao, J. Lai, C. Wang, J. Li, Z. Liu, Boric acid-based dual modulation photoluminescent glucose sensor using thioglycolic acid-capped CdTe quantum dots. J. Anal. Test. 1, 291–297 (2017)
K. Uematsu, T. Ueno, K. Ushimaru, C. Maruyama, Y. Hamano, H. Katano, Colorimetric method to detect ε-poly-L-lysine using glucose oxidase. J. Biosci. Bioeng. 122, 513–518 (2016)
W.-Q. Xie, Y.-X. Gong, K.-X. Yu, Rapid quantitative detection of glucose content in glucose injection by reaction headspace gas chromatography. J. Chromatogr. A 1520, 143–146 (2017)
N.J. Ronkainen, H.B. Halsall, W.R. Heineman, Electrochemical biosensors. Chem. Soc. Rev. 39, 1747–1763 (2010)
Y. Xu, X. Liu, Y. Ding, L. Luo, Y. Wang, Y. Zhang, Preparation and electrochemical investigation of a nano-structured material Ni2+/MgFe layered double hydroxide as a glucose biosensor. Appl. Clay Sci. 52, 322–327 (2011)
C. Mousty, Sensors and biosensors based on clay-modified electrodes—new trends. Appl. Clay Sci. 27, 159–177 (2004)
J. Wang, Electrochemical glucose biosensors. Chem. rev. 108, 814–825 (2008)
T.M. Alfareed, A. Almofleh, S.M. Asiri, J.M. AlGhamdi, S.T. Gunday, E. Cevik, Molybdenum-cobalt micro-nano interface assisted ultrasensitive and selective non-enzymatic glucose biosensor. Microchem. J. 191, 108923 (2023)
B.E. Nugba, N.O. Mousa, A. Osman, A.A. El-Moneim, Non-enzymatic amperometric biosensor with anchored Ni nanoparticles for urinary glucose quantification. Diam. Relat. Mater. 137, 110171 (2023)
Z. Golsanamlou, M. Mahmoudpour, J. Soleymani, A. Jouyban, Applications of advanced materials for non-enzymatic glucose monitoring: from invasive to the wearable device. Crit. Rev. Anal. Chem. 53, 1116–1131 (2023)
A.K. Subramania, S. Sugumaran, P. Sethuramalingam, R. Ramesh, P. Dhandapani, S. Angaiah, NiCo2O4/Ti2NbC2 (double MXene) nanohybrid-based non-enzymatic electrochemical biosensor for the detection of glucose in sweat. Bioprocess Biosyst. Eng. 46, 1755–1763 (2023)
L.Y. Xiong, Y.J. Kim, W.C. Seo, H.K. Lee, W.C. Yang, W.F. Xie, High-performance non-enzymatic glucose sensor based on Co3O4/rGO nanohybrid. Rare Met. 42, 3046–3053 (2023)
A. Mubarakali, S. Gopinath, P. Parthasarathy, U.A. Kumar, A.A. Basha, Highly efficient and sensitive non-enzymatic glucose biosensor based on flower-shaped CuO-colloid nanoparticles decorated with graphene-modified nanocomposite electrode. Measurement 217, 113145 (2023)
M.H. Hassan, C. Vyas, B. Grieve, P. Bartolo, Recent advances in enzymatic and non-enzymatic electrochemical glucose sensing. Sensors 21, 4672 (2021)
H. Jeong, J. Yoo, S. Park, J. Lu, S. Park, J. Lee, Non-enzymatic glucose biosensor based on highly pure TiO2 nanoparticles. Biosensors 11, 149 (2021)
A. Mohammadpour-Haratbar, S. Mohammadpour-Haratbar, Y. Zare, K.Y. Rhee, S.J. Park, A review on non-enzymatic electrochemical biosensors of glucose using carbon nanofiber nanocomposites. Biosensors 12, 1004 (2022)
J. Ahmed, M.A. Rashed, M. Faisal, F.A. Harraz, M. Jalalah, S.A. Alsareii, Novel SWCNTs-mesoporous silicon nanocomposite as efficient non-enzymatic glucose biosensor. Appl. Surf. Sci. 552, 149477 (2021)
P. Si, Y. Huang, T. Wang, J. Ma, Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 3, 3487–3502 (2013)
M. Sivakumar, R. Madhu, S.M. Chen, V. Veeramani, A. Manikandan, W.H. Hung, Low-temperature chemical synthesis of CoWO4 nanospheres for sensitive nonenzymatic glucose sensor. J. Phys. Chem. C 120, 17024–17028 (2016)
Q. Balouch, Z.H. Ibupoto, G.Q. Khaskheli, R.A. Soomro, S.M.K. Sirajuddin, V.K. Deewani, Cobalt oxide nanoflowers for electrochemical determination of glucose. J. Elect. Materials. 44, 3724–3732 (2015)
A.M. Azharudeen, R. Karthiga, M. Rajarajan, A. Suganthi, Fabrication, characterization of polyaniline intercalated NiO nanocomposites and application in the development of non-enzymatic glucose biosensor. Arab. J. Chem. 13, 4053–4064 (2020)
X. Kang, Z. Mai, X. Zou, P. Cai, J. Mo, A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 363, 143–150 (2007)
F. Xiao, F. Zhao, D. Mei, Z. Mo, B. Zeng, Nonenzymatic glucose sensor based on ultrasonic-electrodeposition of bimetallic PtM (M Ru, Pd and Au) nanoparticles on carbon nanotubes–ionic liquid composite film. Biosens. Bioelectron. 24, 3481–3486 (2009)
Y. Xiao, L. Hou, M. Wang, R. Liu, L. Han, M. Nikolai, S. Zhang, C. Cheng, K. Hu, Noninvasive glucose monitoring using portable GOx-Based biosensing system. Anal. Chim. Acta 1287, 342068 (2024)
A.K. Manna, P. Guha, S.K. Srivastava, S. Varma, Non-enzymatic glucose sensors based on electrodeposited CuxO–ZnO composite nanostructures. J. Mater. Sci. Mater. Electron. 35, 1–12 (2024)
Q. Fan, X. Li, H. Dong, Z. Ni, T. Hu, ZIF-67 anchored on MoS2/rGO heterostructure for non-enzymatic and visible-light-sensitive photoelectrochemical biosensing. Biosensors 14, 38 (2024)
M. Zhou, Glucose electrochemical biosensors: research progress and challenges. In Third International Conference on Biological Engineering and Medical Science (ICBioMed2023), 12924, 354–359, SPIE. (2024).
M. Govindaraj, A. Srivastava, M.K. Muthukumaran, P.C. Tsai, Y.C. Lin, B.K. Raja, J. Rajendran, V.K. Ponnusamy, J.A. Selvi, Current advancements and prospects of enzymatic and non-enzymatic electrochemical glucose sensors. Int. J. Biol. Macromol. 253, 126680 (2023)
H. Çiftçi, E. Alver, F. Çelik, A.Ü. Metin, U. Tamer, Non-enzymatic sensing of glucose using a glassy carbon electrode modified with gold nanoparticles coated with polyethyleneimine and 3-aminophenylboronic acid. Microch. Acta. 183, 1479–1486 (2016)
C. Chen, Q. Xie, D. Yang, H. Xiao, Y. Fu, Y. Tan, Recent advances in electrochemical glucose biosensors a revi. Rsc. Adv. 3, 4473–4491 (2013)
F.Y. Liu, D.N. Ding, Y.-R. Wang, S.X. Liu, C. Peng, F. Shen, Icariin as a potential anticancer agent a review of its biological effects on various cancers. Front. Pharmacol. (2023). https://doi.org/10.3389/fphar.2023.1216363
H. Zhu, L. Li, W. Zhou, Z. Shao, X. Chen, Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 4, 7333–7349 (2016)
I.H. Yeo, D.C. Johnson, Anodic response of glucose at copper-based alloy electrodes. J. Elec. Anal. Chem. 484, 157–163 (2000)
N. Mohamad Nor, N.S. Ridhuan, K. Abdul Razak, Progress of enzymatic and non-enzymatic electrochemical glucose biosensor based on nanomaterial-modified electrode. Biosensors 12, 1136 (2022)
A. Şavk, H. Aydın, K. Cellat, F. Şen, A novel high performance non-enzymatic electrochemical glucose biosensor based on activated carbon-supported Pt–Ni nanocomposite. J. Mol. Liq. 300, 112355 (2020)
A. Taşaltın, T.A. Türkmen, N. Taşaltın, S. Karakuş, Highly sensitive non-enzymatic electrochemical glucose biosensor based on PANI: β12 Borophene. J. Mater. Sci. Mater. Electron. 32, 10750–10760 (2021)
A. Taşaltın, Glucose sensing performance of PAN: β-rhombohedral borophene based non-enzymatic electrochemical biosensor. Inorg. Chem. Commun. 133, 108973 (2021)
J. Lillo-Ramiro, J.M. Guerrero-Villalba, M.D.L. Mota-González, F.S. Aguirre-Tostado, G. Gutiérrez-Heredia, I. Mejía-Silva, A. Carrillo-Castillo, Optical and microstructural characteristics of CuO thin films by sol–gel process and introducing in non-enzymatic glucose biosensor applications. Optik 229, 166238 (2021)
M.H. Fahmy Taha, H. Ashraf, W. Caesarendra, A brief description of cyclic voltammetry transducer-based non-enzymatic glucose biosensor using synthesized graphene electrodes. Appl. Syst. Innov. 3, 32 (2020)
F. Mollarasouli, M.R. Majidi, K. Asadpour-Zeynali, Enhanced activity for non-enzymatic glucose biosensor by facile electro-deposition of cauliflower-like NiWO4 nanostructures. J. Taiwan Inst. Chem. Eng. 118, 301–308 (2021)
M. Khan, V. Nagal, U.T. Nakate, M.R. Khan, A. Khosla, R. Ahmad, Engineered CuO nanofibers with boosted non-enzymatic glucose sensing performance. J. Electrochem. Soc. 168, 067507 (2021)
A.S. Agnihotri, A. Varghese, M. Nidhin, Transition metal oxides in electrochemical and bio sensing: a state-of-art review. Appl. Surf. Sci. Adv. 4, 100072 (2021)
S. Ding, T. Zhu, J.S. Chen, Z. Wang, C. Yuan, X.W.D. Lou, Controlled synthesis of hierarchical NiO nanosheet hollow spheres with enhanced supercapacitive performance. J. Mater. Chem. 21, 6602–6606 (2011)
B. Varghese, M. Reddy, Z. Yanwu, C.S. Lit, T.C. Hoong, G. Subba Rao, Fabrication of NiO nanowall electrodes for high performance lithium ion battery. Chem. Mate. 20, 3360–3367 (2008)
B. Wang, J.S. Chen, Z. Wang, S. Madhavi, X.W. Lou, Green synthesis of NiO nanobelts with exceptional pseudo-capacitive properties. Adv. Energy Mater. 2, 1188–1192 (2012)
R. Manigandan, T. Dhanasekaran, A. Padmanaban, K. Giribabu, R. Suresh, V. Narayanan, Bifunctional hexagonal Ni/NiO nanostructures: influence of the core–shell phase on magnetism, electrochemical sensing of serotonin, and catalytic reduction of 4-nitrophenol. Nano. Adv. 1, 1531–1540 (2019)
R.S. Kate, S.A. Khalate, R.J. Deokate, Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: a review. J. Alloys Compd. 734, 89–111 (2018)
R. Eder, Electronic structure of NiO Antiferromagnetic transition and photoelectron spectra in the ordered phase. Phy. Rev. B 91, 245146 (2015)
F.D. Speck, K.E. Dettelbach, R.S. Sherbo, D.A. Salvatore, A. Huang, C.P. Berlinguette, On the electrolytic stability of iron-nickel oxides. Chem 2, 590–597 (2017)
R.D. Smith, M.S. Prévot, R.D. Fagan, S. Trudel, C.P. Berlinguette, Water oxidation catalysis electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing iron, cobalt, and nickel. J. Am. Chem. Soc. 135, 11580–11586 (2013)
C. Xia, X. Yanjun, W. Ning, Facile synthesis of NiO nanoflowers and their electrocatalytic performance. Sens. Actuators B Chem. 153, 434–438 (2011)
M. Youcef, B. Hamza, H. Nora, B. Walid, M. Salima, B. Ahmed, A novel green synthesized NiO nanoparticles modified glassy carbon electrode for non-enzymatic glucose sensing. Microchem. J. 178, 107332 (2022)
D. Mishra, R. Zhou, M.M. Hassan, J. Hu, I. Gates, N. Mahinpey, Bitumen and asphaltene derived nanoporous carbon and nickel oxide/carbon composites for supercapacitor electrodes. Sci. Rep. 12, 4095 (2022)
T. Zahra, K. Shahzad Ahmad, C. Zequine, R. Gupta, A. Thomas, M.A. Malik, S. Iram, Y.A. ElBadry, Z.M. El-Bahy, Electrochemical trapping of meta-stable NiO consolidated ZnO/PdO by biomimetic provenance for the employment of clean energy generation. Mater. Sci. Semicond. Proc. 150, 106867 (2022)
S. Trafela, J. Zavašnik, S. Šturm, K.Ž Rožman, Formation of a Ni (OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta 309, 346–353 (2019)
W. Wang, Z. Zhao, Q. Lei, W. Zhang, P. Li, W. Zhang, Hierarchically Au-functionalized derived ultrathin NiO nanosheets for highly sensitive electrochemical hydrazine detection. Appl. Surf. Sci. 542, 148539 (2021)
R. Ramasamy, K. Ramachandran, G.G. Philip, R. Ramachandran, H.A. Therese, Design and development of Co3O4/NiO composite nanofibers for the application of highly sensitive and selective non-enzymatic glucose sensors. RSC Adv. 5, 76538–76547 (2015)
S.J. Li, Y. Xing, L.L. Hou, Z.Q. Feng, Y. Tian, J.M. Du, Facile synthesis of NiO/CuO/reduced graphene oxide nanocomposites for use in enzyme-free glucose sensing. Int. J. Electrochem. Sci. 11, 6747–6760 (2016)
K. Ghanbari, F. Ahmadi, NiO hedgehog-like nanostructures/Au/polyaniline nanofibers/reduced graphene oxide nanocomposite with electrocatalytic activity for non-enzymatic detection of glucose. Analyt. Biochem. 518, 143–153 (2017)
J. Arguello, H.A. Magosso, R. Landers, V.L. Pimentel, Y. Gushikem, Synthesis, characterization and electroanalytical application of a new SiO2/SnO2 carbon ceramic electrode. Electrochim. Acta 56, 340–345 (2010)
M. Imran Din, A. Rani, Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles a green adeptness. Int. J. Anal. Chem. (2016). https://doi.org/10.1155/2016/3512145
K. Petcharoen, A. Sirivat, Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 177, 421–427 (2012)
F. Jia, L. Zhang, X. Shang, Y. Yang, Non-aqueous sol–gel approach towards the controllable synthesis of nickel nanospheres, nanowires, and nanoflowers. Adv. Mater. 20, 1050–1054 (2008)
B.A. Abbasi, J. Iqbal, Z. Khan, R. Ahmad, S. Uddin, A. Shahbaz, Phytofabrication of cobalt oxide nanoparticles from Rhamnus virgata leaves extract and investigation of different bioactivities. Microsc. Res. Tech. 84, 192–201 (2021)
S. Uddin, L.B. Safdar, S. Anwar, J. Iqbal, S. Laila, B.A. Abbasi, Green synthesis of nickel oxide nanoparticles from Berberis balochistanica stem for investigating bioactivities. Molecules 26, 1548 (2021)
J. Iqbal, B.A. Abbasi, T. Mahmood, S. Kanwal, B. Ali, S.A. Shah, Plant-derived anticancer agents: a green anticancer approach. Asian Pac. J. Trop. Biomed. 7, 1129–1150 (2017)
J. Iqbal, B.A. Abbasi, R. Batool, T. Mahmood, B. Ali, A.T. Khalil, Potential phytocompounds for developing breast cancer therapeutics nature’s healing touch. Eur. J. Pharmacol. 827, 125–148 (2018)
A. Kar, A.K. Ray, Synthesis of nano-spherical nickel by templating hibiscus flower petals. J. Nanosci. Nanotech. 2, 17–20 (2014)
H. Jung, R. Gupta, E. Oh, Y. Kim, C. Whang, Vibrational spectroscopic studies of sol–gel derived physical and chemical bonded ORMOSILs. J. Non-cryst. Solids 351, 372–379 (2005)
C.S. Ferreira, P.L. Santos, J.A. Bonacin, R.R. Passos, L.A. Pocrifka, Rice husk reuse in the preparation of SnO2/SiO2 nanocomposite. Mater. Rese. 18, 639–643 (2015)
H. H. Radamson , A. Hallén , I. Sychugov, A. Azarov, Analytical Methods and Instruments for Micro- and Nanomaterials, Springer Cham, (2023).
S. Yousaf, S. Zulfiqar, M.N. Shahi, M.F. Warsi, N.F. Al-Khalli, M.F.A. Aboud, Tuning the structural, optical and electrical properties of NiO nanoparticles prepared by wet chemical route. Ceram. Internat. 46, 3750–3758 (2020)
J. Tauc, Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 3, 37–46 (1968)
K.E. Toghill, L. Xiao, M.A. Phillips, R.G. Compton, The non-enzymatic determination of glucose using an electrolytically fabricated nickel microparticle modified boron-doped diamond electrode or nickel foil electrode. Sens. actuators B Chem. 147, 642–652 (2010)
Y. Zhang, L. Su, D. Manuzzi, H.V. de los Monteros, W. Jia, D. Huo, C. Hou, Y. Lei, Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosen. Bioelect. 31, 426–432 (2012)
M. Li, X. Bo, Z. Mu, Y. Zhang, L. Guo, Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically reduced graphene oxide film as a nonenzymatic glucose sensor. Sens. Actuators B Chem. 192, 261–268 (2014)
Y. Ni, J. Xu, Q. Liang, S. Shao, Enzyme-free glucose sensor based on heteroatom-enriched activated carbon (HAC) decorated with hedgehog-like NiO nanostructures. Sens. Actuators B Chem. 250, 491–498 (2017)
Z. Deng, H. Long, Q. Wei, Z. Yu, B. Zhou, Y. Wang, High-performance non-enzymatic glucose sensor based on nickel-microcrystalline graphite-boron doped diamond complex electrode. Sens. Actuators B Chem. 242, 825–834 (2017)
L. Sheng, Z. Li, A. Meng, Q. Xu, Ultrafast responsive and highly sensitive enzyme-free glucose sensor based on a novel Ni(OH)2@ PEDOT-rGO nanocomposite. Sens. Actuators B Chem. 254, 1206–1215 (2018)
Y. Zhang, Y. Liu, L. Su, Z. Zhang, D. Huo, C. Hou, CuO nanowires based sensitive and selective non-enzymatic glucose detection. Sens. Actuators B Chem. 191, 86–93 (2014)
J. Xu, N. Xu, X. Zhang, P. Xu, B. Gao, X. Peng, Phase separation induced rhizobia-like Ni nanoparticles and TiO2 nanowires composite arrays for enzyme-free glucose sensor. Sens. Actuators B Chem. 244, 38–46 (2017)
P. Salazar, V. Rico, A.R. González-Elipe, Nickel–copper bilayer nanoporous electrode prepared by physical vapor deposition at oblique angles for the non-enzymatic determination of glucose. Sens. Actuators B Chem. 226, 436–443 (2016)
M. Youcef, B. Hamza, H. Nora, B. Walid, M. Salima, B. Ahmed, A novel green synthesized NiO nanoparticles modified glassy carbon electrode for non-enzymatic glucose sensing. Microch. Jou. 178, 107332 (2022)
Acknowledgments
The authors would like to gratefully acknowledge the Higher Education Commission Pakistan for partial support under the project NRPU/8350/8330. We also extend our sincere appreciation to the Researchers Supporting Project Number (RSP2024R79) at King Saud University, Riyadh, Saudi Arabia, and Ajman University, Grants ID: DRG ref. 2023-IRG-HBS-2 (RESHUSC-001), RTG-2023-HBS-1 (Phase 1).
Author information
Authors and Affiliations
Contributions
Ihsan Ali Mahar, did the material synthesis and partial electrochemical measurements. Aneela Tahira, did XRD analysis did wrote the draft. Mehnaz Parveen, did partial electrochemical measurements. Ahmed Ali Hullio, did optical analysis. Muhammad Ali Bhatti, did XRD measurement. Elmuez Dawi, did the partial electrochemical analysis. Akram Ashames, did partial drafting of the manuscript and validated the electrochemical results. Ayman Nafady, did partial drafting of the manuscript. Riyadh H. Alshammari, did EIS analysis. Brigitte Vigolo, did SEM and EDS analysis. Kezhen Qi, did ECSA analysis. E. Mustafa, did FTIR analysis. L.M. A. Saleem, Overall proofread the electrochemical results. Zafar Hussain Ibupoto, did main supervision, conceptualized the work, and wrote the first draft of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing interests in the resented research work.
Ethical approval
There were no human and animal studies involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahar, I.A., Tahira, A., Parveen, M. et al. Glucose sensing via green synthesis of NiO–SiO2 composites with citrus lemon peel extract. J Mater Sci: Mater Electron 35, 490 (2024). https://doi.org/10.1007/s10854-024-12156-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12156-9