Abstract
In this work, the effect of Ag doping process (directly and as a nanoparticle) into the Y3Fe5O12 (YIG) thin films on the structural, morphological, optical and magnetic properties was studied in detail. Ag-doped YIG thin films were grown on thermally oxidized Si substrates by following sol–gel and spin-coating methods. All films were crystallized without cracks by two-stages heat treatment process. The XRD patterns confirm the formation of YIG peaks, and metallic Ag peaks which settled into the structure without bonding with the YIG components. In both Ag doping processes, the coercive field (Hc) and saturation magnetization (Ms) values of the samples containing 3% Ag are significantly lower than the other samples. The Ms values of 5% Ag doped samples were found the highest in both series. The Ms values of the films between in-plane and out-of-plane measurement increased with the Ag concentration. The optical measurements indicate an absorption peak in the range of 0–4 eV in both sample series and the optical band gap of the films decreased with the Ag doping due to its metallic characteristic. The ferromagnetic resonance measurements indicate that the lowest FMR linewidth as 92 Oe is seen in the 1% Ag-doped YIG sample. The relatively cheap and easy production of the used method and additive material may enable the widespread the usage of Ag-doped YIG thin films in magneto-optical devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the most important material type at the intersection of studies on magnetic and optical properties of materials is magnetic ceramics. Yttrium Iron Garnet (Y3Fe 5O12; YIG) is a magnetic ceramic material that finds wide application area due to its high electrical resistance, excellent gyromagnetic performance, narrow resonance linewidth in microwave region, low magnetization damping value, good saturation magnetization and high-radiation stability [1,2,3,4,5,6,7,8,9]. The magnetic and optical properties of YIG are due to the interactions between its sublattices. The Y3+ ions are considered as non-magnetic while Fe3+ ions are magnetic in the main lattice of YIG. The sublattice structures of YIG occupied as three Fe3+ ions in tetrahedral with 24 (d) sites and octahedral with 16 (a) sites correspond to the magnetization of YIG. The O2− ions include 96 (h) sites in the YIG lattice.
Many studies on YIG are based on the replacement of Y3+ ions or Fe3+ ions with other ions like Dy, Bi, Ce, Ga, Al [10,11,12,13,14,15]. These studies showed that the substitution of the transition metals supplied a large contribution to the magnetic moment from the orbital motion of Y and Fe. The contribution of yttrium’s orbital motion is opposite to that of its spin. Due to the orbital effect, it makes the total magnetic moment of the yttrium atom parallel to the magnetic moment of iron. Therefore, instead of using an alternative doping for yttrium and iron atoms, it would be more logical to dope without disturbing the YIG crystal structure. Silver (Ag) is a noble metal having wide range of optical applications [16]. It is cheaper than other noble metals because it is common in nature. Various methods like liquid phase epitaxy (LPE) [2], magnetron sputtering [17], pulsed laser deposition (PLD) [4] have been used for the deposition of YIG thin films. But, these systems require vacuum for the deposition of YIG films. Vacuum-free methods such as sol–gel dip coating [18] and spin-coating methods [19] can also be used to grow YIG films because of their low cost and large area deposition ability. On the other hand, the gadolinium gallium garnet (GGG) substrate was used to deposit the YIG films due to their good matching. However, the gadolinium as a rare-earth metal limits its commercially usage because of its high cost. To replace GGG substrate by other cheap substrates and to integrate YIG thin films with semiconductor applications while maintaining the YIG’s crystal system, the films should be grown on silicon or related substrates.
So far, various experimental studies on the magneto-optical effect for Au/Bi:YIG granular film [20], a nano-onion with multicore–shell structure [21] and Au particles embedded Bi:YIG film [22] were discussed in the literature. However, the effect of Ag doping process into the YIG thin films grown on Si substrate is needed to be addressed for its possible magnetic and optical applications. Therefore, in this study, we comprehensively investigated the effect of Ag-doping process (directly and as a nanoparticle) and their concentrations (1%, 3% and 5%) into the Y3Fe5O12 (YIG) thin films grown on Si substrate on the structure, morphology, optical and magnetic properties.
2 Experimental procedure
Ag (directly) and Ag-nanoparticle-doped YIG thin films were produced by sol–gel and spin-coating method. Figure 1 shows the schematically illustrated production process of the nanoparticle, thin films and heat treatment processes. The whole processes from particle synthesize and characterizations are explained as follows.
2.1 Ag nanoparticle synthesize
Ag nanoparticles was first synthesized by polyol method and then its various wt% (1%, 3% and 5%) were added to YIG solution to produce thin films (see Fig. 1a). To synthesize nanoparticles, PVP and AgNO3 were dissolved separately in stoichiometric proportions in ethylene glycol (EG) at 160 °C and 600 rpm for 30 min. Then, the PVP solution was transferred dropwise into the AgNO3 solution. PVP is used here to prevent aggregation in solution [23]. Then, the synthesized nanoparticles were washed with ethanol and acetone by following centrifugation at 10.000 rpm. The spherical shape of Ag nanoparticles has been observed in SEM images shown in Fig. 1. The average particle size was found around 40 nm.
2.2 Ag-doping processes
The chemical solution of YIG films was prepared as follows; in appropriate stoichiometric ratios of yttrium (III) nitrate tetrahydrate (N2O6Y·4H2O) with purity 99.99% (Aldrich), iron (III) nitrate nanohydrate (Fe(NO3)3·9H2O) with purity 98.0% (Alfa Aesar) were separately dissolved in 2-methoxyethanol. Then, both solutions were mixed and allowed to stir for 48 h at 50 °C. (100) oriented Si is the most commonly used substrate in microelectronic and magnetic applications [24]. However, YIG growth on a direct Si substrate is a challenge due to the complexity of the Y–Fe–O phase diagram and the lattice mismatch between Si and YIG [25]. Therefore, we used thermally oxidized Si substrate to be a buffer layer between YIG and Si [26]. Two-stages spin-coating process was used to grow YIG films that the solution is dropped on the substrate and it releases to accelerate to 1000 rpm in 10 s and then accelerate immediately to 3000 rpm for 59 s. Then, grown films were dried at 110 °C for 30 min in ambient atmosphere to burn off the organic solvents. The Ag doping process was applied as (1) for Ag nanoparticle doped YIG; the synthesized Ag nanoparticles were added into the YIG solution (see Fig. 1a), (2) for Ag doped YIG; the appropriate amount of silver nitrate (AgNO3) was dissolved in 2-methoxyethanol and then added into the YIG solution (see Fig. 1b). The mixture was stirred for 48 h. After that the same growing process was used as we did in YIG thin films. The mixture was stirred for 48 h. After that the same growing process was used as we did in YIG thin films.
2.3 Heat treatment process
Figure 1c shows a representative graph of the applied two-stage heat treatment process. The films were heated to 750 °C with a heating rate of 2 °C/min and kept at this temperature for 3 h. Afterwards, the films were cooled to 500 °C with a cooling rate of 1 °C/min and kept at this temperature for 3 h. Finally, it was cooled to room temperature with a cooling rate of 1 °C/min. Thus, it is aimed that no crack formation is observed in the films thanks to the gradual cooling process.
2.4 Characterization techniques
X-Ray Diffraction (XRD) with Cu-Kα radiation was used to determine the phase formation and the structural properties of the films. The microstructure and surface properties were studied by Scanning Electron Microscope (SEM). Physical Properties Measurement System (PPMS) with Vibrating Sample Magnetometer (VSM) head was used to characterized the magnetic properties of samples at room temperature. Ferromagnetic resonance (FMR) spectra were measured at 9 GHz frequency at room temperature. Optical characteristics of films were analyzed by using UV–Vis measurement technique.
3 Results and discussions
3.1 Microstructural analysis
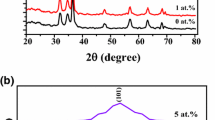
To observe the effect of Ag doping process (directly and as a nanoparticle) into the YIG thin films on the crystal structure, XRD measurements were performed in between 20º-60º and then analyzed by using X-Pert High-Score Plus software. Figure 2a–d show the XRD profile of YIG, 1%, 3% and 5% Ag-doped YIG thin films, respectively. The XRD patterns of the YIG thin film shown in Fig. 2a exhibit characteristic YIG diffraction peaks having garnet structure that are (400), (420) and (422) located at 2θ = 28.83°, 32.33°, and 35.54°, respectively. It confirms that YIG films grown on Si/SiO2 substrate are crystallized in a single-phase cubic garnet structure. For the Ag doped YIG films, in addition to the YIG crystalline peaks, a metallic Ag peak with a (200) orientation at 2θ = 38.12° and 44.35° is clearly seen in the XRD patterns. A peak corresponding to the Si(200) is observed at approximately 33° in the XRD patterns of all samples which it comes from the substrate. In the XRD profiles of samples, the observing both characteristic YIG and metallic Ag peaks indicate that the films are crystalline in the desired structure. The crystallite sizes of undoped YIG and Ag-doped YIG thin films were calculated with the Debye–Scherrer formula,
where D is the crystallite size, λ is the wavelength of radiation (Cu Kα = 1,5405 Å), β is the full width at half maximum of the intensity peak and θ is the angle of diffraction peak. The calculated crystallite sizes of all samples are given in Table 1. The mean crystallite sizes of the YIG film phase were calculated in the range of 31–62 nm (see Table 1). The differences in crystallite sizes of the samples might come from the sol–gel synthesize and/or film coating process. On the other hand, the XRD patterns of YIG films doped via 1%, 3% and 5% Ag nanoparticles are shown in Fig. 2e–g, respectively. Similar XRD patterns with the directly Ag-doped YIG films were observed in Ag-NPs doped YIG thin films. The metallic Ag peak (002) is observed in the XRD patterns of the Ag-NPs doped YIG thin films. The intensities of metallic Ag peaks are higher than the directly Ag doped samples. As seen in the XRD graphs in Fig. 2, the peak intensity of the YIG thin film decreased with Ag doping that it indicates the deterioration of crystalline quality of the YIG via Ag doping. Ag may induce a strain in the doped crystal lattice that can change the lattice periodicity and reduce crystallinity of the YIG [27].
Figure 3a–f show the SEM images of the 1%, 3%, 5% Ag-doped YIG, 1%, 3%, 5% Ag-NPs-doped YIG thin films, respectively. The SEM images indicate smooth surface morphology without cracks and nucleation. The grain morphology changes by the Ag concentration in both directly Ag-doped and Ag-NPs-doped samples (See Fig. 3). However, there are some small islands observed in 3% Ag-doped YIG film. These islands might come from the metallic Ag grains [28]. The island-like agglomerations affects the magnetic characteristic of YIG thin films that it will be discussed below. The cross-sectional SEM images of the films are shown in Fig. 3g. The thicknesses of the films were determined by using cross-sectional SEM images and tabulated in Table 1. A nonlinear thickness dependence with the Ag concentration behavior was observed in the all films. While the thickness of undoped YIG thin film was determined as 92 nm, the thickness of the films increased with the Ag doping.
3.2 Magnetic properties
Magnetic properties of undoped YIG, Ag-doped YIG and Ag-NPs-doped YIG thin films were investigated by using VSM system. The magnetic hysteresis measurements were carried out in two different ways by applying an in-plane (IP) and out-of-plane (OOP) magnetic field to the film surface at room temperature. Figure 4a–f show the IP and OOP hysteresis curves of the YIG, Ag-doped and Ag-NPs-doped YIG thin films. It is noted that the diamagnetic contribution from the substrate has been subtracted from the raw hysteresis curves. Although all hysteresis curves are similar each other, the magnetic parameters such as saturation magnetization (Ms) and anisotropy field (Ha), are different. Moreover, it can be deduced from the hysteresis curves that while the 1% Ag-doped film has in-plane easy axis magnetic anisotropy, the magnetic characteristics of the films become isotropic when the Ag content is increased. A very low coercive field (Hc) for all samples (see Table 2) is observed that it indicates the samples are soft magnetic (ferromagnetic) at room temperature. In both Ag doping processes, the Hc and Ms values of the samples containing 3% Ag are significantly lower than the other samples. This might be related to the its lowest crystal quality and thickness as it was discussed above. The Ms values of 5% Ag doped samples were found the highest in both series. The Ms values of the films between in-plane and out-of-plane measurement increased with the Ag concentration. Magnetic anisotropy energy values (K) are calculated using K = MsHa/2 relation and tabulated in Table 2. According to the calculated K values, although the anisotropy energies of the samples are in the same order, when Ag is added to the YIG film, a nonlinear change is observed in the K values. It is obviously observed that the Ag doping, independent to the doping process, makes the films as magnetically isotropic. Ms, Hc, Ha and K values extracted from hysteresis curves are tabulated in Table 2.
Ferromagnetic resonance (FMR) spectra of Ag-doped and Ag nanoparticle doped YIG thin films were measured at 9 GHz frequency at room temperature. FMR plots of Ag-doped and Ag NP-doped YIG thin films are shown in Fig. 5a, b, respectively. According to the graphs of Ag-doped samples in Fig. 5a, the lowest FMR linewidth as 92 Oe is seen in the 1% Ag-doped YIG sample, while the highest FMR linewidth as 193 Oe is seen in the 3% Ag-doped sample. Similar behavior has been observed in the Ag NPs doped YIG thin films as shown in Fig. 5b. The lowest (highest) FMR linewidth is determined as 72 Oe (122 Oe) in the 1% (3%) Ag NPs doped YIG thin film. Although the trend in the FMR linewidth with the Ag content is same in both Ag-doped and Ag-NPs doped YIG films, the FMR linewidths is lower in Ag-NPs doped samples than directly Ag doped samples. The reason for this is thought to be due to the contribution of the surface plasmonic resonance effect of Ag nanoparticles to the ferromagnetic resonance of the films. The FMR results are in good agreement with the SEM images shown in Fig. 3. Because the magnetic homogeneity of magnetic thin films are associated with surface defects, porosity, microstructure and grain size [29,30,31], the surface of the 3% Ag-doped and 3% Ag-NPs doped films is more porous than the other samples (see Fig. 3b, e). As a result of porous surface, the surface defects may have caused the broadening in the FMR linewidth. These results are also consistent with film thicknesses (see Table 1). As it is known, FMR linewidth decreases with increasing film thickness [32, 33]. Figure 5c shows a comparison of our experimental results with the similar works in the literature. The FMR linewidth of our thin film coated on the Si substrate by the sol–gel method are much lower than the films (348 Oe) grown in vacuum required systems [34]. In previous studies, YIG thin films were grown on many different substrates. Some of the most commonly used of these substrates are gadolinium gallium garnet (GGG) [35,36,37], Si-Si/SiO2 [33, 38, 39] and quartz [32, 40, 41]. GGG substrate is used to grow YIG films because the mismatch between them is less than others. But, because the GGG substrate contains rare-earth metal (Gd), it is expensive. In addition, the use of GGG substrate leads to attenuation of magnon and microwave excitations in the cavity spintronic field, especially at cryogenic temperatures [42]. Quartz and Si–Si/SiO2 substrates are cheaper materials than GGG. However, the difference between the coefficient thermal expansion of the quartz substrate (CTE: 0.5 × 10−6/°C) and the coefficient thermal expansion of the YIG thin film (CTE: 10 × 10−6/°C) leads to crack formation in the thin films. Moreover, the usage of quartz as a substrate limits the usage of YIG in semiconductor technology. The results in this work are interesting in terms of comparable FMR results with the YIG films grown on GGG, quartz and Si substrates. Our films were grown both a cheap method (sol–gel and spin-coating vacuum-free method) and a cheap/commercially usable substrate (Si/SiO2).
3.3 Optic properties
The optical properties of YIG thin films, which are widely used in magneto-optical applications, are as important as their magnetic properties. One of the most important optical properties of thin films is their absorption coefficient (α). Therefore, the effect of the Ag doping ratios and process on the absorption coefficient of the YIG thin film were studied by performing UV–VIS measurements. The α values of the samples were determined by using absorbance data α = 2.303A/t where A is absorbance and t is thickness of the films. Figure 6a, b show the absorption coefficient graphs of Ag-doped and Ag-NPs-doped YIG thin films at various Ag concentrations, respectively. The absorption coefficient graph of the undoped YIG thin film can be seen in the inset of Fig. 6a. According to this graph, the undoped YIG thin film grown on Si substrate does not show an absorption peak between 0 and 4 eV energy values. However, absorption peaks of around 2 eV are exhibited in the samples (see Fig. 6a, b). These peaks are corresponding to the absorbance behavior of Ag [43]. The absorption coefficient of Ag-NPs-doped YIG thin films was lower than that of Ag-doped YIG samples. This might be related to the localized surface plasmon polaritons effect of Ag NPs. Both graphs show a decrease in the absorption coefficient with increasing Ag doping. The decrease in the absorption coefficient with increasing silver doping has been reported in some previous studies [28].
Next, we determined the optical band gap of the samples by using following equation and the Tauc plot;
where ν, A, h and Eg represent the frequency of the incoming light, energy-independent constant, Planck-constant, optical band gap, respectively. The n in Eq. (2) determines the material’s optical transition type. The n value can take on the values 2 for indirect band gap and 1\2 for direct band gap depending on the nature of the transmission. Based on the optical data and Eq. (2), we plot (αhν)1/2 versus photon energy (hν) of Ag-doped and Ag-NPs-doped YIG thin films in Fig. 6c, d, respectively. Then, we calculated the energy band gap of the films by finding the intersections from a linear fit in the steadily increasing region in Fig. 6c, d. The experimental bandgap value of the undoped YIG thin film was calculated as approximately 2.9 eV which is consistent with the literature [44]. The optical bandgap values of the films decreased with the increasing Ag content in the samples regardless of the Ag-doping process. The variation of the band gap values with the Ag content is shown in Fig. 7. The reduction in Eg might be related to the metallic characteristic of Ag that it reduces the band gap of the whole structure. Jollybose et al. doped Ag into tungsten oxide film at similar doping rates to those in this study and found that the band gap decreased with increasing Ag doping. They suggested that the reason for this effect could be quantum confinement in the films [45]. Similar studies have also shown that the band gap narrowed with increasing Ag doping [46, 47]. Marotti et al. suggested that the reason for this effect is that the absorption behavior and band gap of nanostructured materials change with the quantum size effect (QSE) [48].
Absorption coefficient of a Ag doped YIG thin films (The inset represents absorption coefficient pattern of YIG thin film) b AgNPs doped YIG thin films. Plot of (αhν)1/2 vs. photon energy (hν) of c Ag doped YIG thin films, d Ag NPs doped YIG thin films (The inset represents plot of (αhν)1/2 vs. photon energy (hν) of YIG thin films)
4 Conclusions
In summary, the effect of Ag, as a noble metal, doping process into the YIG grown on thermally oxidized Si substrates by vacuum-free sol–gel spin-coating method on the structure, morphology, magnetic and optical properties of YIG thin films was studied in detail. XRD patterns indicate that the films are crystallized in cubic crystal structure consisting with the reference YIG patterns as well as observing metallic Ag crystal peaks. The magnetic measurement results showed that the Ag doping makes the magnetically isotropic of the films. In both series, Ms values of 3% Ag added samples were the lowest, while 5% Ag added samples had the highest Ms values. The coercive field (Hc) values of the Ag-NP doped samples are smaller than the same doping ratios in directly Ag-doped samples. The ferromagnetic resonance line widths of Ag-NP doped samples are narrower than those of directly Ag doped samples at the same rates. The minimum FMR linewidth was observed as 92 Oe in the 1% Ag-doped YIG sample. Indirect band gap values of the films decreased from 2.9 eV to 1.9 eV with the doping of Ag in both series. According to the data obtained from our study, doping Ag as nanoparticles into the YIG structure has more advantageous than directly Ag doping process. This can provide many advantages such as lower energy loss in the demagnetization process, fast magnetic response, high magnetic sensitivity and the ability to operate at high frequency. Ag-doped YIG thin films grown on Si substrate via vacuum-free method can be widely used in magneto-optical devices due to their comparable magnetic and optical properties and low-production cost.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
F.W. Aldbea, N.B. Ibrahim, M. Yahya, Effect of adding aluminum ion on the structural, optical, electrical and magnetic properties of terbium doped yttrium iron garnet nanoparticles films prepared by sol–gel method. Appl. Surf. Sci. 321, 150–157 (2014)
J.C. Butler et al., Microwave and magneto-optic properties of bismuth‐substituted yttrium iron garnet thin films. J. Appl. Phys. 67(9), 4938–4940 (1990)
Z. Capku, F. Yildiz, Spin wave modes observation in YIG thin films with perpendicular magnetic anisotropy. J. Magn. Magn. Mater. (2021). https://doi.org/10.1016/j.jmmm.2021.168290
C. Hauser et al., Yttrium iron garnet thin films with very low damping obtained by recrystallization of amorphous material. Sci. Rep. (2016). https://doi.org/10.1038/srep20827
S. Khanra et al., Structural and magnetic studies of Y3Fe5–5xMo5xO12. J. Magn. Magn. Mater. 369, 14–22 (2014)
V. Sharma et al., YIG based broad band microwave absorber: a perspective on synthesis methods. J. Magn. Magn. Mater. 439, 277–286 (2017)
A. Sposito et al., Combinatorial pulsed laser deposition of doped yttrium iron garnet films on yttrium aluminium garnet. J. Appl. Phys. (2014). https://doi.org/10.1063/1.4864134
S.R. Wadgane et al., Ferro- and magneto-electric characteristics in YFeO3–Y3Fe5O12 nanocomposites. J. Magn. Magn. Mater. 457, 103–109 (2018)
J. Wang et al., Effect of manganese addition on the microstructure and electromagnetic properties of YIG. J. Rare Earths. 29(6), 562–566 (2011)
A.B. Bhosale et al., Influential incorporation of RE metal ion (Dy3+) in yttrium iron garnet (YIG) nanoparticles: magnetic, electrical and dielectric behaviour. Ceram. Int. 46(10), 15372–15378 (2020)
H.I. Hayashi, S. Vasa, N.J. Yoshitake, T. Ueda, K. Yokoyama, S. Higuchi, S. Takeshita, Fabrication of Bi-doped YIG optical thin film for electric current sensor by pulsed laser deposition. Appl. Surf. Sci. (2002). https://doi.org/10.1016/S0169-4332(02)00364-1
S.U. Higuchi, K. Yahiro, F. Nakata, Y. Uetsuhara, Fabrications of cerium-substituted YIG thin films for magnetic field sensor by pulsed-laser deposition. IEEE Trans. Magn. 37(4), 2451–2453 (2021)
A. Ikesue et al., Giant faraday rotation in heavily Ce-doped YIG bulk ceramics. J. Eur. Ceram. Soc. 40(15), 6073–6078 (2020)
M.I. Khan et al., Magnetic behavior of Ga doped yttrium iron garnet ferrite thin films deposited by sol–gel technique. Ceram. Int. 46(17), 27318–27325 (2020)
M.A. Musa et al., Influence of aluminum substitution on microstructural, electrical, dielectric, and electromagnetic properties of sol–gel synthesized yttrium iron garnet (YIG). AIP Adv. (2020). https://doi.org/10.1063/1.5111894
R. Shyamala, L.N.G. Devi, Surface plasmon resonance effect of Ag metallized SnO2 particles: exploration of metal induced gap states and characteristic properties of ohmic junction. Surf. Interface Anal. 52(6), 374–385 (2020)
J. Lian et al., Annealing effects on the microstructure and magnetic properties of Y3Fe5O12 films deposited on Si/SiO2 substrates by RF magnetron sputtering. Ceram. Int. 43(10), 7477–7481 (2017)
O. Opuchovic et al., Cerium doping and cerium aluminium co-doping effects on the sol–gel processing of Y3Fe5O12 (YIG): Bulk and thin films. Solid State Sci. (2020). https://doi.org/10.1016/j.solidstatesciences.2019.106065
M. Akyol et al., Structural, magnetic and optical properties of Au/YIG, YIG/Au and Au/YIG/Au multilayer thin film stacks. J. Magn. Magn. Mater. (2020). https://doi.org/10.1016/j.jmmm.2019.165704
S. Tomita et al., Magneto-optical Kerr effects of yttrium-iron garnet thin films incorporating gold nanoparticles. Phys. Rev. Lett. 96(16), 167402 (2006)
M. Abe, T. Suwa, Surface plasma resonance and magneto-optical enhancement in composites containing multicore-shell structured nanoparticles. Phys. Rev. B (2004). https://doi.org/10.1103/PhysRevB.70.235103
R. Fujikawa et al., Contribution of the surface plasmon resonance to optical and magneto-optical properties of a Bi:YIG-Au nanostructure. J. Appl. Phys. (2008). https://doi.org/10.1063/1.2829036
D. Kim, S. Jeong, J. Moon, Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology. 17(16), 4019–4024 (2006)
P. Zaumseil, High-resolution characterization of the forbidden Si 200 and Si 222 reflections. J. Appl. Crystallogr. 48(Pt 2), 528–532 (2015)
Y. Zhang et al., Growth of phase pure yttrium iron garnet thin films on silicon: the effect of substrate and postdeposition annealing temperatures. IEEE Trans. Magn. 51(11), 1–4 (2015)
X. Zhou et al., Effect of post-annealing temperature on the microstructure and magnetic properties of ce:YIG thin films deposited on Si substrates. Appl. Surf. Sci. 253(4), 2108–2112 (2006)
S.R. Chalana, V. Ganesan, V.P. Mahadevan, Pillai, Surface plasmon resonance in nanostructured Ag incorporated ZnS films. AIP Adv. (2015). https://doi.org/10.1063/1.4933075
P.O. Offor et al., Structural, morphological and optical properties of spray-formed silver-doped zinc sulphide thin films. Optik. 185, 519–528 (2019)
L.K.C.S. Assis et al., The FMR line width and the structure in YIG films deposited by MOD on silicon (1 0 0). J. Magn. Magn. Mater. (2023). https://doi.org/10.1016/j.jmmm.2023.170388
A.V. Nazarov et al., Near theoretical microwave loss in hot isostatic pressed (hipped) polycrystalline yttrium iron garnet. J. Appl. Phys. 94(11), 7227–7234 (2003)
T.N. Thi et al., Morphology-dependent spin Seebeck effect in yttrium iron garnet thin films prepared by metal-organic decomposition. Ceram. Int. 47(12), 16770–16775 (2021)
B. Bhoi, Bulk-like magnetization and improved microwave properties of polycrystalline YIG (Y3Fe5O12) films grown on quartz substrates by pulsed laser deposition. J. Alloys Compd. 797, 523–528 (2019)
A. Delgado et al., Combining the sol gel method and spin coating to obtain YIG films with low FMR linewidth on silicon (100) substrate. Mater. Res. Express (2018). https://doi.org/10.1088/2053-1591/aaaff8
H. Zheng et al., Preparation of low ferromagnetic resonance linewidth yttrium iron garnet films on silicon substrate. Appl. Surf. Sci. 307, 661–664 (2014)
B. Bhoi et al., Effect of annealing on the structural and FMR properties of epitaxial YIG Thin films grown by RF magnetron sputtering. IEEE Trans. Magn. 54(11), 1–5 (2018)
Q. Guo et al., Target grain size dependence of the morphology, crystallinity and magnetic properties of yttrium iron garnet films. Ceram. Int. 45(3), 3414–3418 (2019)
H. Zheng et al., Tens of micron-thick, crack-free yttrium iron garnet films on a Gd3Ga5O12 substrate based on the layer by layer growth method. J. Mater. Sci. Mater. Electron. 29(14), 11790–11794 (2018)
X. Guo et al., Growth and characterization of yttrium iron garnet films on Si substrates by chemical solution deposition (CSD) technique. J. Alloys Compd. 671, 234–237 (2016)
Y.-M. Kang et al., Magnetic properties of YIG (Y3Fe5O12) thin films prepared by the post annealing of amorphous films deposited by Rf-magnetron sputtering. J. Appl. Phys. (2005). https://doi.org/10.1063/1.1855460
B. Bhoi, M.S. Diware, Preferred-oriented polycrystalline Y3Fe5O12 films grown on quartz with low microwave loss. J. Am. Ceram. Soc. 104(6), 2423–2427 (2021)
B. Bhoi et al., Effect of oxygen pressure on the magnetic properties of yttrium-iron-garnet thin films made by pulsed laser deposition. IEEE Mangn. Lett. 7, 1–4 (2016)
A. Krysztofik, S. Ozoglu, E. Coy, Magnetization damping in nanocrystalline yttrium iron garnet thin films grown on oxidized silicon. IEEE Mangn. Lett. 12, 1–5 (2021)
M. Venkatesham et al., A novel green synthesis of silver nanoparticles using gum karaya: characterization, antimicrobial and catalytic activity studies. J. Cluster Sci. 25(2), 409–422 (2013)
O.I. Gorbatov et al., Magnetic exchange interactions in yttrium iron garnet: a fully relativistic first-principles investigation. Phys. Rev. B (2021). https://doi.org/10.1103/PhysRevB.104.174401
R. Jolly Bose et al., Effect of silver incorporation in phase formation and band gap tuning of tungsten oxide thin films. J. Appl. Phys. (2012). https://doi.org/10.1063/1.4768206
D.R. Sahu, Studies on the properties of sputter-deposited Ag-doped ZnO films. Microelectron. J. 38(12), 1252–1256 (2007)
H. Xue et al., Influence of Ag-doping on the optical properties of ZnO films. Appl. Surf. Sci. 255(5), 1806–1810 (2008)
R.E. Marotti et al., Crystallite size dependence of band gap energy for electrodeposited ZnO grown at different temperatures. Sol. Energy Mater. Sol. Cells. 90(15), 2356–2361 (2006)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by Çukurova University Scientific Research Council under project number: FDK-2019-11492. N.D. would like to acknowledge TUBITAK-BIDEB 2211 C “The Scientific and Technological Research Council of Turkey” for her financial support during this work.
Author information
Authors and Affiliations
Contributions
ND: Performed experiments/data collection, data analysis and interpretation, drafted the paper, provided grammatical revisions to manuscript, provided revisions to scientific content of manuscript. MA: Performed experiments/data collection, data analysis and interpretation, provided grammatical revisions to manuscript, provided revisions to scientific content of manuscript. FK: Data analysis and interpretation, provided grammatical revisions to manuscript, provided revisions to scientific content of manuscript. AE: Data analysis and interpretation, edited the paper, provided grammatical revisions to manuscript, provided revisions to scientific content of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors stated that did not have conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demiryürek, N., Akyol, M., Karadağ, F. et al. Effect of Ag-doping process into the yttrium iron garnet (Y3Fe5O12) thin films on the structural, magnetic and optical properties. J Mater Sci: Mater Electron 35, 392 (2024). https://doi.org/10.1007/s10854-024-12124-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12124-3