Abstract
A unique Al/Terp-Pyr/p-Si/Al diode structure that has not before been presented was introduced in this paper. Utilizing capacitance-conductance-frequency (C-G-f) characteristics in the frequency range of 20 Hz− 1.5 MHz for four temperatures of 300 K, 325 K, 350 K, and 375 K, admittance analysis was carried out to disclose the impedance and dielectric properties of the diode. The appearance of interface states at the Terp-Pyr/p-Si interface leads to an increase in capacitance values at low frequencies. Using complex impedance spectroscopy, the impedance characteristics of the Al/ Terp-Pyr/p-Si/Al Schottky diode were examined. The fabricated diode’s dielectric, modulus, and ac conductivity properties were investigated in the same frequency and temperature range. It has been revealed that while \({\epsilon }^{{\prime }}\) decreases up to high frequencies for all temperatures, it grows with temperature in the low-frequency zone. It becomes apparent that while the real component (M’) of modulus grows for all temperatures from low to high frequencies, it decreases at high frequencies as temperature rises. remarkably adjustable temperature, voltage, and frequency tuning of the dielectric constants \(({\epsilon }^{{\prime }},{{\epsilon }^{{\prime }}}^{{\prime }})\) and dielectric loss tangent (tanδ). Additionally, the equivalent circuit of the Al/ Terp-Pyr/p-Si/Al Schottky diode and Cole-Cole diagrams were explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A knowledge of the electrical characteristics of Schottky structures is essential for the development of electronic devices [1, 2]. The ideality factor and Schottky barrier height of the devices are two main parameters that are significantly influenced by the interface layer between the Schottky contact and inorganic semiconductor and interface states [3]. A very important factor in metal-organic material-semiconductor (MOmS)-type Schottky structures is the surface uniformity and stability of organic compounds. The development of an organic layer between the semiconductor and metal contact is crucial for these devices’ dependability and performance [4, 5]. Several studies in literature support the potential use of organic semiconducting materials in a wide range of electronic devices, including light emitting diodes [6], photodetectors [7], lasers [8], field-effect transistors [9], thin-film batteries [10], solar cells [11], supercapacitors [12], and so on.
The devices with organic interfaces have several benefits, such as the ability to cover a wide area, flexibility, and low cost, even though some work is needed because of their poor performance [13]. Organic semiconductors are difficult to use in high frequency applications because they naturally have lower mobility than inorganic semiconductors. There are, however, some reports of devices made of organic materials that have been tested over a large frequency range [5, 14,15,16]. Measurements of transient capacitance [17, 18] and impedance spectroscopy [19,20,21] have been used to examine the effects of interfacial polarizations, ac conductivity, and dielectric relaxation. The frequency response of impedance spectroscopy also indicated that the Schottky structures could usually be defined by one or a couple of parallel capacitor-resistor network unions [19,20,21]. Yahia et al. [16] have investigated the dielectrical, impedance, and electrical conduction properties of the methyl orange (MO) at various temperatures and frequencies. They found that all properties are strongly dependent on frequency and temperature. Karadaş et al. [15] have reported the electric modulus, complex dielectric, and electrical conductivity properties of the graphene-PVA at various frequencies. Their results showed that GR doped PVA is a good material for making ultra-capacitor compared to other inorganic structures. Shehata et al. [5] have investigated the complex impedance, complex dielectric, and electric modulus properties of the perylene-66 in the temperature range from 303 to 383 K and the frequency range from 100 Hz to 2 MHz. They found that the temperature has strong influence on the conductivity and impedance spectroscopy of the sample particularly at intermediate (p-Si/Al interface) and lower (Perylene-66/p-Si interface) frequencies. Kagatikar and their groups used pyrene for electron donor material in bulk heterojunction solar cells [22]. Two different materials as pyrene as electron donor linked to phenyl group (PC1) and pyrene as electron donor linked to fluorophenyl group (PC2) were synthesized and taken capacitance and conductance measurement. Values of dielectric constant of PC1 and PC2 were − 6 and − 12 at 5 kHz, respectively [22]. Figgemeier et al. examined the dielectric constant of terpyridine in different solvents these values were 80.1, 38.8, and 9.08 for H2O, CH3CN, and CH2Cl2, respectively [23].
In this work, we aim to design and synthesize a new molecule containing terpyridine and pyrene (Terp-Pyr) and provide the findings of our investigation into the equivalent circuit, dielectric constants, and relaxation dynamics, including timings, process, and mechanism, of a novel form of Schottky structure. The synthesis and electrical transport properties of the Terp-Pyr compound are described in the current study with this objective in mind. Capacitance, conductance, and impedance measurements have been used to examine electrical properties. In the frequency range of 20 Hz to 1.5 MHz and in the temperature range of 300–375 K, an extensive impedance, dielectric, and modulus response of Terp-Pyr has been made. Due to its simple precipitation and stability on the silicon surface, as well as the temperature- and frequency-dependent characteristics of the generated structure, Terp-Pyr organic semiconductor can be employed particularly in organic-electronic devices.
2 Experimental details
2.1 Synthesis processes of Terp-Pyr
4’-(4-(bromomethyl)phenyl)-2,2’:6’,2’’-terpyridine (2) and 4’-(4-(azidomethyl)phenyl)-2,2’:6’,2’’-terpyridine (3) were synthesized using the methods described in the literature. Here, the pyrene-substituted 2,2’:6’,2’’-terpyridine compound (Terp-Pyr) is synthesized for the first time. The synthesis of Terp-Pyr is shown in Scheme. 1.
4’-(4-(azidomethyl)phenyl)-2,2’:6’,2’’-terpyridine (3) (0.28 mmol) and 1-ethynylpyrene (4) (0.28 mmol) were dissolved in the minimum amount of DMF. After stirring the solution under an argon atmosphere until it was completely transparent, sodium ascorbate (0.28 mmol) was added. The solution was then left under an argon atmosphere for 5 min. The solution was then mixed with CuSO4.5H2O (0.28 mmol), and again placed in an argon atmosphere for 5 min. Then, the reaction mixture was stirred at 80 °C for 6 h. The formed precipitate was filtered, and the crude product was purified by column chromatography. Fraction containing Terp-Pyr was collected then the solvent was removed under reduced pressure (yield: 82%). 1H NMR (400 MHz, (CH3OD/CDCl3)) H 8.68–8.60 (m, 6 H), 8.53 (d, 1H, J = 8 Hz), 8.19–7.88 (m, 13H), 7.56 (d, 2 H, J = 8 Hz), 7.39 (t, 2 H, J = 4 Hz), (Ar-CH); 5.77 (s, 2 H); 13C NMR (100 MHz, CDCl3) 155.76, 155.70, 149.52, 148.79, 148.78, 139.84, 137.56, 135.56, 131.44, 131.29, 130.78, 129.89, 128.56, 128.29, 128.06, 127.93, 127.27, 127.18, 126.11, 125.46, 125.18, 124.91, 124.81, 124.63, 124.59, 124.34, 124.24, 123.26, 121.87, 54.01 ppm (Fig. 1).
2.2 Fabrication of Al/Terp-Pyr/p-Si/Al diode
Al/Terp-Pyr/p-Si/Al Schottky structure was prepared on a thickness of 525 μm, (100) orientation, 20 Ω-cm resistivity and boron doped p type Si substrate with the help of both spin coating and thermal evaporation methods. The Si wafer was cleaned using the Radio Corporation of America (RCA) method and then to eliminate the native oxide before Terp-Pyr and metal deposition it is also dipped with dilute HF (H2O:HF, 10:1) for 40 s. Ohmic contact on the back of the n-Si crystal was fabricated by thermal evaporating of aluminum (Al, 200 nm) metal, and then the crystal was a temperature treatment at 570 °C for 3 min in N2 atmospheres. To prepare a solution of organic material, 10 mg of Terp-Pyr was dissolved in 10 mg of chloroform. A spin coater operating at 1500 rpm for 60 s was used to deposit Terp-Pyr solution to the Si crystal. Vacuum evaporated aluminum (Al) metal with thickness of 200 nm was used to deposit rectifying contact to the Terp-Pyr layer. As a result, Al/ Terp-Pyr/p-Si/Al Schottky structure was fabricated. A HP 4192 A LF impedance analyzer system was used to measure the complex impedance-frequency (Z*–f) capacitance-frequency (C–f) and conductance-frequency (G–f) features of the Al/Terp-Pyr/p-Si/Al Schottky structure. The electrical properties of the fabricated diode were studied by frequencies ranging from 300 Hz to 1.5 MHz and temperatures ranging from 300 to 375 K with 25 K ranges.
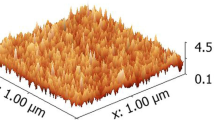
Understanding the surface morphology of an organic layer is crucial for enhancing the performance of any semiconductor-based device during device fabrication. The surface morphology properties of the Terp-Pyr layer grown on a p type silicon semiconductor were studied by scanning electron microscopy (SEM). Figure 2a displays the SEM image of the Terp-Pyr organic layer fabricated on p-Si. Figure 2b displays the cross-sectional SEM image of the Terp-Pyr/p-Si structure. It is evident that the surface morphology of Terp-Pyr organic film is completely homogeneous and contains spherical shaped, with an average size of 0.3–0.5 μm. The thickness of Terp-Pyr organic compound on Si was an average cross-sectional size of 56–61 nm.
3 Results and discussion
3.1 Impedance spectroscopy
An impedance is called total resistance within an electrical circuit with several electrical circuit elements when an AC voltage is applied [24]. The composition of samples, electrodes, interfaces between samples and their electrodes, the morphology of samples are factors that affect the impedance [25]. The impedance (Z) is represented the function of frequency by impedance spectroscopy, and it has two components as a real part and an imaginary part [26]. Z’ is the real part and Z’’ is the imaginary part of impedance in Eq. 1.
The impedance spectroscopy measurements were taken between 20 Hz and 1.5 MHz for four temperatures of 300 K, 325 K, 350 K, and 375 K. The variation of the real part of impedance with frequency is given in Fig. 3. Z ' shows different behaviors in three different regions according to the frequency. While Z’ decreased with increasing frequency within the middle frequency region in the measuring range, it showed saturation behavior in the low and high frequency region. The long-range movements of the charge carriers are shown in the low frequency region. The charge carriers hop while their neighboring carriers relax at their site because of this movement. The localized movements of the charge carriers are shown in the middle frequency region. They can unsuccessfully hop while their neighbors relax at their sites due to this movement. Because of these movements, DC resistivity is dominated in the low frequency region, however, ac conductivity appears in the middle frequency region. Thus Z ' depended on the frequency at the middle frequency region. Saturation behavior in high frequency regions occurs with the resistive behavior of the grains of the material [27, 28]. Also, High Z’ values at low frequencies may be due to space charge effects [29]. When the behavior of Z’ was examined that depend on temperature, it decreased with increasing frequency within the middle frequency region in the measuring range, but it did not show regular behavior in the low frequency region. In high frequency regions, it is saturated for all temperatures.
The variation of the imaginary part of impedance with frequency is given in Fig. 4a. This graph describes the relaxation peaks caused by some different constituent parameters such as grain, grain boundary, and electrodes [30]. Relaxation peaks appeared at all temperatures for the fabricated device. While one peak was observed at 300 and 325 K, two peaks were observed at 350 and 375 K, and the second peak appeared at the low frequency region for these temperatures. The presence of two peaks is evidence of two different relaxation times in the system. The peaks in the lower frequency region are associated with grain boundaries, and the peaks in the higher frequency region are associated with grains [31]. All relaxation peaks shifted toward the high frequency region and the intensity of the peaks decreased with increasing temperature. This situation can be said that the relaxation process depends on temperature and the relaxation time decreased because of the accelerated hopping rate of charge carriers with increasing temperature [32]. Also, the expansion of the peaks with increasing temperature is a proof that the relaxation time depends on the temperature in the fabricated device [33].
The variation of Z’’/Z’’max with f/fmax is given in Fig. 4b. Observed peaks for all temperatures overlap perfectly. This may be proof that the relaxation process is the same for all temperatures [34].
The activation energy was calculated used of peaks of relaxation time (τ) at high frequency. Relaxation times were determined for each temperature. Figure 5 shows a graph of lnτ versus 1000/T for fabricated devices from which activation energy (Ea) was calculated using the Arrhenius equation [35]:
τ0 is pre-exponential factor, kb is the Boltzmann constant, and T is the temperature. Ea and τ0 were calculated 0.165 eV and 2.44 × 10−8 s, respectively.
The graph of the real part versus the imaginary part of the impedance is called the Nyquist plot. Figure 6 represents the Nyquist plot for the device of Al/ Terp-Pyr/p-Si/Al at different temperatures. As a semicircular was observed for 300 K, a second semicircular appeared at 325 K. It was seen that the two semicircles are separated from each other at 350 and 375 K. This confirms the presence of two relaxation mechanisms at two interfaces (Terp-Pyr/p-Si and Al/p-Si) in 350 and 375 K and the presence of one a relaxation mechanism at an interface (Terp-Pyr/p-Si). Generally, the semicircular at the high frequency region is related to the grain effect, while the semicircular at the low frequency region is related to the grain boundary effect [36]. Referring to this statement, while the grain effect decreased with increasing temperature, the grain boundary effect increased with increasing temperature. The radius of the semicircular almost decreased with increased temperature, this is the negative temperature coefficient of resistivity, which is a typical semiconductor behavior [37]. Also, the observation of different semicircles can be associated with different relaxation times of the material [38]. This statement supports the observation of two different peaks in the imaginary part of impedance versus frequency.
Nyquist curves were fitted to a suitable equivalent circuit given in the inset of Fig. 6 to examine the behavior of the device under an AC voltage. The circuit consists of two parallel resistance (R)-constant phase (CPE) elements connected in series resistance. The first parallel circuit shows the grain effect, while the second parallel circuit depicts the grain boundary effect. CPE is defined as given in Eq. (3).
ω is the angular frequency (\(\omega = {\text{2pf}}\)), the unit of Q is the unit of capacitance, and n takes a value between − 1 and 1. The behavior of the CPE in the material changes according to the value it takes. Most common situations: behaves like a capacitor, a resistance, and a coil when n takes a value of 1, 0, and − 1, respectively. The analytic equation of the equivalent circuit is given in Eqs. (4), (5), and (6).
The values of all circuit elements fitted according to the analytical equations for each temperature are given in Table 1. While series resistance (Rs) and grain resistance (Rg) decreased with increased temperatures, grain boundary resistance (Rgb) increased. Both grain Q (Qg) and grain boundary Q (Qgb) decreased with increased temperature.
3.2 The dielectric spectroscopy
The dielectric constant (\(\epsilon\)) describes some information about the polarization mechanisms of materials in an electric field [39]. This constant depends on capacitance and can be calculated from the measured capacitance. Also, it depends on total polarization mechanisms such as electronic, ionic, orientation, and space charge [40, 41]. The dielectric constant consists of two components as imaginary and real parts. The real part of the dielectric constant (ε’) is related to energy stores since the imaginary part of the dielectric constant (ε’’) is related to energy losses [42]. They are given in Eqs. (7), (8), and (9).
C is measured capacitance values, G is measured conductance values, ω is the angular frequency, A is the contact area of the fabricated sample, d is the thickness of the polymer materials, and ε0 is the dielectric constant (8.85 × 10−12 F/m). According to Eq. (8), the real part of the dielectric constant is directly proportional to the capacitance.
The capacitance and conductance measurements were taken between 300 Hz and 1.5 MHz for four temperatures of 300 K, 325 K, 350 K, and 375 K. The plots of depending on the frequency of the reel part of the dielectric constant are given in Fig. 7 for different temperatures. ε’ reduced with increasing frequency at the mid frequency region, while it saturated at the high frequency region. The decrease in dielectric constant is associated with the reduction of polarization effects [43]. Saturation of ε’ at the high frequency region is based on the inability of the charges to follow the AC signal. This observed behavior in the fabricated sample is a typical dielectric behavior that is described by Koop’s theory [44]. The real part of the dielectric constant increased with increasing temperature, especially at low frequency regions. Contrary to the frequency-dependent behavior of the dielectric constant, it increases with increasing temperature. Increasing the real part of the dielectric constant with increasing temperature is interesting in improved dipole polarization [44].
The dielectric loss tangent (tanδ) is related to dielectric relaxations and resonance. This is represented in Eq. (10).
Figure 8 shows the frequency-dependent behavior of the dielectric loss tangent for different temperatures. The dielectric loss tangents reduced with the increased temperature at low frequencies region for all temperatures. A peak disappeared at 1.2 kHz for the last temperature (375 K), while any peaks were not observed for other temperatures. This observed peak may relate to the conduction mechanism. This is attributed to the resonance between the hopping frequency of the charge carriers and the frequency of the applied AC signal [45]. At the high frequency region dielectric loss saturated for all temperatures. It decreased with increasing temperature at low frequency regions. Similar behavior exists in previous literature [46].
The graph of ln ε’’ versus lnω for the fabricated device at different temperatures is given in Fig. 9. There is a relationship between the imaginary part of the dielectric constant and angular frequency, as in Eq. (11).
A is a constant and m is the frequency power factor. The value of m can be determined with the slope of the graph of ln ε’’ versus lnω and values of m were negative at all temperatures. The values were 0.50, 0.47, 0.44, and 0.33 at different temperatures for the fabricated device. The relationship between m and temperature is given in Eq. 12.
k b is the Boltzmann constant (8.62 × 10−5 eV/K), T is temperature and Wm barrier height. The barrier height is determined by the slope of the graph of m versus temperature, and its value is 1.86 eV.
3.3 The modulus spectroscopy
Electrical modulus spectroscopy is used to study electrical characterization, such as relaxation time and polarization mechanism due to grain, grain boundary, and electrode effects [47]. Likewise, with complex impedance, the electrical modulus (M) has two components such as real (M ‘) and imaginary (M ‘’) electrical modulus. The formalism of the two components of the electrical modulus is given in Eqs. 13 and 14.
The variation of the real part of the electrical modulus with frequency is given in Fig. 10. Real part of the electrical modulus increased with increasing frequency. It increased rapidly increased rapidly at the mid - frequency region, whereas it was saturated at the low and high frequency region. The reasons for the steady values of modulus values are the high mobility of the charge carriers and the low contribution of the Maxwell Wagner Sillars polarization effect [48]. For all temperatures, the real part of the electrical modulus was nearly zero at low frequency region, this behavior is due to the negligible electrode polarization [49]. The real part of the modulus decreased with increasing temperature, the reason for this statement could be the short-range migration of the charge carriers [50].
Figure 11 (a) represents the plots of the real part of modulus versus frequency at different temperatures for the fabricated device. Relaxation peaks were observed for all temperatures. These peaks shifted to high frequency region, and the intensity of the peaks decreased and broadened with increasing temperature. The peaks at the low frequency region represent long-term mobility of the charge carrier while they at the high frequency region represent short-term mobility of the charge carrier. The shift of the peaks to different frequency regions with temperature indicates that the relaxation processes could depend on temperature [51, 52]. Relaxation times were 5.46 × 10−5 s, 4.83 × 10−5 s, 3.37 × 10−5 s, and 1.73 × 10−5 s for 300 K, 325 K, 350 K, and 375 K, respectively. Figure 11 (b) represents the plots of M ‘’/M ‘’max versus f/fmax at all temperatures. All curves overlapped perfectly, and this behavior indicates that the relaxation processes describe the same mechanism at all temperatures [53].
Figure 12 shows the graph of the real part of the electrical modulus versus the imaginary part of the electrical modulus. Likewise, the impedance spectroscopy, electric modulus spectroscopy describes some effect such as grain, grain boundary, or electrode. Unlike the impedance spectroscopy, in modulus spectroscopy, the grain boundary effect is observed at low frequency regions while the grain effect is observed at high frequency regions. One semicircular was observed as dominating all temperatures at the high frequencies’ region, but the second semicircular appeared at 350 and 375 K at the low frequency’s region in the inset of Fig. 12. It can be said that the grain effect decreased with increasing temperature, while the grain boundary effect increased with increasing temperature. Similar results were observed for impedance spectroscopy too. Similar behaviors happened for two techniques and the two different techniques supported each other.
The plots of M ‘’/M ‘’max versus f/fmax and Z ‘’/Z ‘’max versus f/fmax for the fabricated device at different temperatures are given in Fig. 13. If the peak positions of the Z ‘’/Z ‘’max and M ‘’/M ‘’max are overlapped, this might be evidence of delocalized or shorth-range relaxation [54]. The peak positions of the Z ‘’/Z ‘’max and M ‘’/M ‘’max did not overlap, but they were very close according to Fig. 13. If these peaks are not overlapped, the relaxation process is long range. The fact that the peak position of Z ‘’/Z ‘’max was in the lower frequency region than the Peak position of M ‘’/M ‘’max and they do not overlap is evidence of long range and localized relaxation [55]. order to mobilize the localized electron, lattice oscillation is needed. Here, it is assumed that the electrons do not move on their own but move with a hopping motion due to the motion of the lattice oscillation [54].
3.4 The AC conductivity
Electrical conductivity \(\left({\sigma }_{\text{T}}\right)\) occurs in two terms as DC conductivity and AC conductivity. While DC conductivity is dominant at low frequency regions, AC conductivity is dominant at high frequency regions.
The universal power law is given in Eq. (16) but some results obey this equality at mid frequency region, while they do not obey at high frequency region. This situation is named as the jump relaxation model [55]. According to this model, a jump ion has a chance to hop back. Namely, the hopping ion back after an unsuccessful hopping. But if the neighboring ion relaxes according to its position, the ion stays in its new place neighbor ion. Ion hopping is successful at mid frequency regions, but unsuccess hopping numbers are higher than successful hopping numbers at high frequency regions [56,57,58,59,60,61,62]. The new form of the universal power law is given in Eq. (17).
A 1, A2 is constant of depending on temperature, s and r are the frequency exponent. While the parameter of s takes a value of 0 and 1, r is between 0 and 2. s relates to translation hopping, while r relates to localized or reorientation hopping. The plots of the lnσac versus lnω are given in Fig. 14 at different temperatures. Values of r are between 1.41 and 1.28. Values of s take between 0.33 and 0.50 and these values decrease with increasing temperatures. According to the correlated barrier hopping (CBH) theory, s decreases with increasing temperature [56]. This behavior obeys CBH theory and the relationship between the maximum-barrier high and s is given in Eq. (18).
\(\tau _{0}\) is the characteristic relaxation time and its approximate value is 10−12 s. \(W_{m} /k_{b} T \gg \ln \left( {\omega \tau _{0} } \right)\) thus Eq. (18) becomes Eq. (19).
The graph of 1-s versus temperature is given in the inset of Fig. 14 and Wm was calculated as 0.293 eV.
4 Conclusions
In the present work, Al/ Terp-Pyr/p-Si Schottky structure has been prepared to see the influence of Terp-Pyr organic interlayer on the impedance, dielectric, modulus, and ac conductivity characteristics. We have studied frequency-dependent response in Al/Terp-Pyr/p-Si structure of Schottky diodes for four different temperatures. The equivalent circuit of the fabricated structure that is two parallel resistances (\(R\))-constant phase (CPE) elements connected in series resistance (\({R}_{s}\)) was able to be contracted from the analysis of Cole–Cole plot.
As 350 and 375 K Cole-Cole plots indicated two semicircles over the investigated range of the frequency, 300 and 325 K Cole-Cole plots indicated one a semicircle. This confirms the presence of two relaxation mechanisms at two interfaces (Terp-Pyr/p-Si and Al/p-Si) in 350 and 375 K and the presence of one a relaxation mechanism at an interface (Terp-Pyr/p-Si). The Cole–Cole analysis showed different relaxation mechanisms for the different temperature ranges. The reduction in radius of the 300-350 K Cole-Cole curves is due to a temperature dependent mechanism associated with the relaxation process. In addition, for all studied temperatures, the ac conductivity values increase with increasing frequency can be ascribed to correlated barrier hopping (CBH) theory. The values of \(\varepsilon ^{\prime}\) and \(\sigma _{{AC~}}\)are increased as \(M^{{~'}}\) is decreased with increasing temperature. Also, the values of \(\sigma _{{AC~}}\) and \(M^{{~'}}\)are increased as \(\varepsilon ^{\prime}\) is decreased with increasing frequency.
Moreover, this studied structure Al/Terp-Pyr/p-Si studies as a Schottky device, and by controlling the voltage, frequency, and temperature, the needed parameters from this structure can be regulated to make it proper for any required applications; this is obvious from investigated impedance, dielectric, modulus, and ac conductivity properties. In conclusion, Terp-Pyr organic compound can be utilized in different applications due to its dielectric behavior.
Data availability
All data generated and analyzed during this study are included in this publication.
References
B. Sharma, Metal-semiconductor Schottky barrier junctions and their applications (Springer, Heidelberg, 2013)
S.M. Sze, K.K. Ng, Physics of semiconductor devices, 3rd edn. (Wiley, Hoboken, 2007)
E.H. Nicollian, J.R. Brews, MOS (metal oxide semiconductor) physics and technology (Wiley, New Jersey, 2002)
Ç. Bilkan, Y. Azizian-Kalandaragh, Ö. Sevgili, Ş. Altındal, J. Mater. Sci. - Mater. Electron. 30, 20479–20488 (2019)
M.M. Shehata, M.O. Abdel-Hamed, K. Abdelhady, Vacuum. 151, 96–107 (2018)
S. Lee, H. Kim, Y. Kim, InfoMat 3, 61–81 (2021)
J. Liu, Y. Wang, H. Wen, Q. Bao, L. Shen, L. Ding, Solar RRL. 4, 2000139 (2020)
D. Suresh, C.S.B. Gomes, P.S. Lopes, C.A. Figueira, B. Ferreira, P.T. Gomes, R.E. Di Paolo, A.L. Maçanita, M.T. Duarte, A. Charas, J. Morgado, D. Vila-Viçosa, M.J. Calhorda, Chem. Eur. J. 21, 9133–9149 (2015)
Y. Yamashita, Sci. Technol. Adv. Mater. 10, 024313 (2009)
Z. Zhao-Karger, P. Gao, T. Ebert, S. Klyatskaya, Z. Chen, M. Ruben, M. Fichtner, Adv. Mater. 31, 1806599 (2019)
Z. Wang, F. Zhang, J. Wang, X. Xu, J. Wang, Y. Liu, Z. Xu, Chin. Sci. Bull. 57, 4143–4152 (2012)
A. Mauger, C. Julien, A. Paolella, M. Armand, K. Zaghib, Materials. 12, 1770 (2019)
L. Ma, J. Ouyang, Y. Yang, Appl. Phys. Lett. 84, 4786–4788 (2004)
S. Demirezen, A. Eroğlu, Y. Azizian-Kalandaragh, Ş. Altındal, J. Mater. Sci. - Mater. Electron. 31, 15589–15598 (2020)
S. Karadaş, S.A. Yerişkin, M. Balbaşı, Y. Azizian-Kalandaragh, J. Phys. Chem. Solids. 148, 109740 (2021)
I.S. Yahia, M.S. Abd El-sadek, F. Yakuphanoglu, Dyes Pigm. 93, 1434–1440 (2012)
A.J. Campbell, D.D.C. Bradley, E. Werner, W. Brütting, Org. Electron. 1, 21–26 (2000)
H.L. Gomes, P. Stallinga, H. Rost, A.B. Holmes, M.G. Harrison, R.H. Friend, Appl. Phys. Lett. 74, 1144–1146 (1999)
A.J. Campbell, D.D.C. Bradley, D.G. Lidzey, J. Appl. Phys. 82, 6326–6342 (1997)
M. Meier, S. Karg, W. Riess, J. Appl. Phys. 82, 1961–1966 (1997)
J. Scherbel, P.H. Nguyen, G. Paasch, W. Brütting, M. Schwoerer, J. Appl. Phys. 83, 5045–5055 (1998)
S. Kagatikar, D. Sunil, D. Kekuda, M.N. Satyanarayana, S.D. Kulkarni, Y.N. Sudhakar, A.K. Vatti, A. Sadhanala, Mater. Chem. Phys. 293, 126839 (2023)
E. Figgemeier, L. Merz, B.A. Hermann, Y.C. Zimmermann, C.E. Housecroft, H.J. Güntherodt, E.C. Constable, J. Phys. Chem. B 107, 1157–1162 (2003)
N.O. Laschuk, E.B. Easton, O.V. Zenkina, RSC Adv. 11, 27925–27936 (2021)
L. Manjakkal, E. Djurdjic, K. Cvejin, J. Kulawik, K. Zaraska, D. Szwagierczak, Electrochim. Acta. 168, 246–255 (2015)
A. Seçkin, H. Koralay, S. Cavdar, N. Turan, N. Tuğluoğlu, ECS J. Solid State Sci. Technol. 11, 083014 (2022)
J. Datta, M. Das, A. Dey, S. Sil, R. Jana, S. Halder, P. Das, P.P. Ray, Mater. Chem. Phys. 213, 23–34 (2018)
S. Jana, J. Datta, S. Maity, B. Thakurta, P.P. Ray, C. Sinha, Cryst. Growth. Des. 21, 5240–5250 (2021)
K. Chandra Babu Naidu, N. Suresh Kumar, G. Ranjith Kumar, S. Naresh Kumar, J. Aust Ceram. Soc. 55, 541–548 (2018)
R.K. Panda, R. Muduli, S.K. Kar, D. Behera, J. Alloys Compd. 615, 899–905 (2014)
M. Idrees, M. Nadeem, M.M. Hassan, J. Phys. D: Appl. Phys. 43, 155401 (2010)
N. Turan, P. Oruç, Y. Demirölmez, A. Seçkin, A.O. Çağırtekin, S. Cavdar, H. Koralay, N. Tuğluoğlu, J. Sol-Gel Sci. Technol. 100, 147–159 (2021)
Y. Wang, Y. Pu, P. Zhang, J. Alloys Compd. 653, 596–603 (2015)
H. Mahamoud, B. Louati, F. Hlel, K. Guidara, Ionics 17, 223–228 (2011)
A. Shukla, R.N.P. Choudhary, A.K. Thakur, J. Phys. Chem. Solids. 70, 1401–1407 (2009)
N. Suresh Kumar, R. Padma Suvarna, Chandra Babu Naidu. Mater. Chem. Phys. 223, 241–248 (2019)
B. Behera, P. Nayak, R.N.P. Choudhary, J. Alloys Compd. 436, 226–232 (2007)
J. Bisquert, L. Bertoluzzi, I. Mora-Sero, G. Garcia-Belmonte, J. Phys. Chem. C 118, 18983–18991 (2014)
R.A. Dorey, Ceramic Thick Films for MEMS and Microdevices, William Andrew (Elsevier, Oxford, 2011)
A.M. Abdelghany, G. El-Damrawi, A.H. Oraby, M.A. Madshal, Phys. B 573, 22–27 (2019)
P. Oruç, N. Turan, Y. Demirölmez, A. Seçkin, Ş. Çavdar, H. Koralay, N. Tuğluoğlu, J. Mater. Sci. - Mater. Electron. 32, 15837–15850 (2021)
Ü. Akın, Ö.F. Yüksel, N. Tuğluoğlu, Silicon 14, 2201–2209 (2022)
I.K. Er, A. Ajjaq, A. Ateş, S. Acar, Bull. Mater. Sci. 45, 212 (2022)
S.F. Mansour, M.A. Abdo, F.L. Kzar, And J. Magn. Magn. Mater. 465, 176–185 (2018)
D.K. Mahato, Mater. Today: Proc. 5, 9191–9195 (2018)
H.M. Kotb, M.M. Ahmad, S.A. Ansari, T.S. Kayed, A. Alshoaibi, Molecules. 27, 779 (2022)
P. Gupta, L.K. Meher, R.N.P. Choudhary, Appl. Phys. A 126, 187 (2020)
A. Rout, S. Agrawal, Ceram. Int. 47, 7032–7044 (2021)
S.F. Chérif, A. Chérif, W. Dridi, M.F. Zid, And a J. Chem. 13, 5627–5638 (2020)
O. Polat, M. Coskun, R. Kalousek, J. Zlamal, B. Zengin Kurt, Y. Caglar, M. Caglar, A. Turut, J. Phys. : Condens. Matter. 32, 065701 (2020)
M.B. Hossen, A.K.M. Akther Hossain, J. Adv. Ceram. 4, 217–225 (2015)
I.K. Er, A.O. Çağırtekin, A. Ajjaq, M.A. Yıldırım, A. Ateş, S. Acar, J. Mater. Sci. - Mater. Electron. 32, 13594–13609 (2021)
A. Shukla, R.N.P. Choudhary, Curr. Appl. Phys. 11, 414–422 (2011)
P. Oruç, N. Turan, S. Cavdar, N. Tuğluoğlu, H. Koralay, J. Mater. Sci.: Mater. Electron. 34(6), 498 (2023)
A. Dutta, T.P. Sinha, J. Phys. Chem. Solids. 67, 1484–1491 (2006)
P.T. Phong, B.T. Huy, Y.I. Lee, I.J. Lee, J. Alloys Compd. 583, 237–243 (2014)
K. Funke, Prog. Solid State Chem. 22, 111–195 (1993)
Y. Badali, Ş. Altındal, İ. Uslu, Progress in Natural Science: Materials International. 28, 325 (2018)
N. Delen, S. Altındal Yerişkin, A. Özbay, Taşçıoğlu, Phys. B Condens Matter 665, 415301 (2023)
A. Tataroǧlu, I. Yücedaǧ, Ş. Altindal, Microelectron. Eng. 85, 1518 (2008)
S. Altındal Yerişkin, E. Erbilen, Tanrıkulu, M. Ulusoy, Mater. Chem. Phys. 303, 127788 (2023)
A. Feizollahi Vahid, S. Alptekin, N. Basman, M. Ulusoy, Şafak Asar, and Altındal. J. Mater. Sci.: Mater. Electron. 34(13), 1–11 (2023)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Pınar Oruç: Conceptualization, Investigation, Methodology, Data analyzing, Validation, Formal analysis, Writing—original draft, Serkan Eymur: Investigation, Resource, Formal analysis, Writing—review and editing, Nihat Tuğluoğlu: Resources, Project administration, Writing—review and editing, Supervision.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oruç, P., Eymur, S. & Tuğluoğlu, N. Investigation of Terp-Pyr/p-Si diode using complex impedance spectroscopy depending on measurement temperatures and frequencies. J Mater Sci: Mater Electron 35, 314 (2024). https://doi.org/10.1007/s10854-024-12029-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12029-1