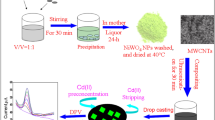
Abstract
Heavy metals are toxic and persistent in the environment and can accumulate in the food chain and potential health hazards to humans and other living organisms. Exposure to heavy metals can cause a range of health effects, including neurological damage, developmental and reproductive problems, and increased risk of certain types of cancer. Thus, monitoring the levels of heavy metal ions in the environment is essential. In these studies, tin nanoparticles (SnNPs) were synthesized using the chemical reduction method to modify the electrode surface on an indium tin oxide (ITO) electrode for heavy metal detection. Two approaches were used in synthesis of SnNPs: one with the presence of polyvinylpyrrolidone (PVP) polymers, namely, SnNP WPVP and the other was performed without a PVP polymer, namely, SnNP WOPVP. The modified electrode was subjected to cyclic voltammetry and square wave anodic stripping voltammetry. The electrode modified with Nafion/100 × SnNP WPVP/ITO showed good performance in the simultaneous detection of Cd2+ and Pb2+ ions and had a linear range of 10–100 ppb, and the low limits of detection for the simultaneous detection of Cd2+ and Pb2+ ions were 1.44 and 1.97 ppb, respectively. The sensitivity of the Nafion/100 × SnNP WPVP/ITO–modified electrode was 0.25 and 0.20 μA/ppb toward Cd2+ and Pb2+ ions, respectively. In the interference study, the modified electrode showed high selectivity towards Cd2+ and Pb2+ ions. The feasibility of using the modified electrode in detecting Cd2+ and Pb2+ ions was tested in a seawater sample. The successful detection of Pb2+ in seawater demonstrated the potential of the modified electrode as a heavy metal ions sensor.
















Similar content being viewed by others
References
G.A. Engwa et al., Mechanism and health effects of heavy metal toxicity in humans (Intech open access, London, 2019), p.13
M. Sebastian, B. Mathew, Ion imprinting approach for the fabrication of an electrochemical sensor and sorbent for lead ions in real samples using modified multiwalled carbon nanotubes. J. Mater. Sci. 53(5), 3557–3572 (2018)
P.B. Tchounwou et al., Molecular, clinical and environmental toxicicology volume 3: environmental toxicology. Mol. Clin. Environ. Toxicol. 101, 133–164 (2012)
H.L. Byers, L.J. McHenry, T.J. Grundl, XRF techniques to quantify heavy metals in vegetables at low detection limits. Food Chem.: X (2019). https://doi.org/10.1016/j.fochx.2018.100001
M. Ahmed et al., Microwave assisted digestion followed by ICP-MS for determination of trace metals in atmospheric and lake ecosystem. J. Environ. Sci. (China) 55, 1–10 (2017)
S. Hassanpoor et al., Ultra-trace determination of arsenic species in environmental waters, food and biological samples using a modified aluminum oxide nanoparticle sorbent and AAS detection after multivariate optimization. Microchim. Acta (2015). https://doi.org/10.1007/s00604-015-1532-6
P. Kumar et al., Progress in the sensing techniques for heavy metal ions using nanomaterials. J. Indust. Eng. Chem. 54, 30–43 (2017)
M. Miyake, Electrochemical functions. Carbon alloys: novel concepts to develop carbon science and technology (Elsevier, Amsterdam, 2003)
M. Ghalkhani, M. Salehi, Electrochemical sensor based on multi-walled carbon nanotubes–boehmite nanoparticle composite modified electrode. J. Mater. Sci. 52(20), 12390–12400 (2017)
J. Barón-Jaimez, M.R. Joya, J. Barba-Ortega, Anodic stripping voltammetry - ASV for determination of heavy metals. J. Phys.: Conf. Ser. (2013). https://doi.org/10.1088/1742-6596/466/1/012023
K. Xu et al., Anodic stripping voltammetry with the hanging mercury drop electrode for trace metal detection in soil samples. Chemosensors 9(5), 107 (2021)
L. Zhou, W. Xiong, S. Liu, Preparation of a gold electrode modified with Au–TiO2 nanoparticles as an electrochemical sensor for the detection of mercury(II) ions. J. Mater. Sci. 50(2), 769–776 (2015)
N. Serrano et al., Coating methods, modifiers and applications of bismuth screen-printed electrodes. TrAC, Trends Anal. Chem. 46, 15–29 (2013)
E.C. Okpara et al., Electrochemical detection of selected heavy metals in water: a case study of African experiences. RSC Adv. 12(40), 26319–26361 (2022)
H. Yang et al., Reliable synthesis of bismuth nanoparticles for heavy metal detection. Mater. Res. Bull. 48(11), 4718–4722 (2013)
Y. He et al., Synthesis of bismuth nanoparticle-loaded cobalt ferrite for electrochemical detection of heavy metal ions. RSC Adv. 10(46), 27697–27705 (2020)
S. Palisoc, R.I.M. Vitto, M. Natividad, Determination of heavy metals in herbal food supplements using bismuth/multi-walled carbon nanotubes/nafion modified graphite electrodes sourced from waste batteries. Sci. Rep. 9(1), 18491 (2019)
B. Wang et al., Simultaneous electrochemical detection of Cd (II) and Pb (II) based on l-cysteine functionalized gold nanoparticles/metal-organic frameworks-graphene oxide nanocomposites. J. Electroanal. Chem. 943, 117573 (2023)
T. Priya et al., Ultra sensitive electrochemical detection of Cd2+ and Pb2+ using penetrable nature of graphene/gold nanoparticles/modified l-cysteine nanocomposite. Chem. Phys. Lett. 731, 136621 (2019)
I. Ivanišević, The role of silver nanoparticles in electrochemical sensors for aquatic environmental analysis. Sensors (2023). https://doi.org/10.3390/s23073692
A.V. Pawar et al., MemSens: a new detection method for heavy metals based on silver nanoparticle assisted memristive switching principle. J. Mater. Sci.: Mater. Electron. 30(12), 11383–11394 (2019)
T.L. Nguyen et al., Platinum nanoflower-modified electrode as a sensitive sensor for simultaneous detection of lead and cadmium at trace levels. J. Chem. 2019, 6235479 (2019)
M.G. Trachioti, J. Hrbac, M.I. Prodromidis, Determination of Cd and Zn with “green” screen-printed electrodes modified with instantly prepared sparked tin nanoparticles. Sens. Actuat. B Chem. 260, 1076–1083 (2018)
Y.Q. Tian, N.B. Li, H.Q. Luo, Simultaneous determination of trace zinc(II) and cadmium(II) by differential pulse anodic stripping voltammetry using a MWCNTs-NaDBS modified stannum film electrode. Electroanalysis 21(23), 2584–2589 (2009)
M. Finšgar, B. Petovar, K. Vodopivec, Bismuth-tin-film electrodes for Zn(II), Cd(II), and Pb(II) trace analysis. Microchem. J. 145, 676–685 (2019)
C. Kokkinos, A. Economou, D. Giokas, Paper-based device with a sputtered tin-film electrode for the voltammetric determination of Cd(II) and Zn(II). Sens. Actuat. B: Chem. (2018). https://doi.org/10.1016/j.snb.2017.12.182
A.T. Singh et al., Paper-based sensors: emerging themes and applications. Sensors (Switzerland) 18(9), 1–22 (2018)
S.W. Tong, K.P. Loh, Graphene properties and application, in Comprehensive hard materials. ed. by V.K. Sarin (Elsevier, Oxford, 2014), pp.565–583
S.S. Chee, J.H. Lee, Synthesis of tin nanoparticles through modified polyol process and effects of centrifuging and drying on nanoparticles. Trans. Nonferrous Met. Soc. China (English Edition) (2012). https://doi.org/10.1016/S1003-6326(12)61791-9
D. Vollath, Agglomerates of nanoparticles. Beilstein J. Nanotechnol. 11, 854–857 (2020)
H.A. Ahmad et al., Effect of PVP as a capping agent in single reaction synthesis of nanocomposite soft/hard ferrite nanoparticles. J. Magn. Magn. Mater. 428, 219–222 (2017)
M.B. Ismail et al., A comparative study on surface treatments in the immobilization improvement of hexahistidine-tagged protein on the indium tin oxide surface. J. Nanomed. Nanotechnol. (2016). https://doi.org/10.4172/2157-7439.1000372
P. Scherrer, Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen (Springer, Berlin, 1912)
V. Uvarov, I. Popov, Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 58(10), 883–891 (2007)
J.I. Langford, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 11(2), 102–113 (1978)
I.A. Safo et al., The role of polyvinylpyrrolidone (PVP) as a capping and structure-directing agent in the formation of Pt nanocubes. Nanoscale Adv. (2019). https://doi.org/10.1039/C9NA00186G
Abeer Jabra Shnoudeh et al., Synthesis, characterization, and applications of metal nanoparticles. Biomaterials and bionanotechnology (Elsevier, Amsterdam, 2019)
V. Selvamani, Stability studies on nanomaterials used in drugs. Micro and nano technologies (Elsevier, Amsterdam, 2019)
Y.Y. Chan et al., Assessments of surface coverage after nanomaterials are drop cast onto electrodes for electroanalytical applications. ChemElectroChem 2(7), 1003–1009 (2015)
I. Jeerapan, S. Poorahong, Review—flexible and stretchable electrochemical sensing systems: materials, energy sources, and integrations. J. Electrochem. Soc. (2020). https://doi.org/10.1149/1945-7111/ab7117
D. Gounden, S. Khene, N. Nombona, Electroanalytical detection of heavy metals using metallophthalocyanine and silica-coated iron oxide composites. Chem. Pap. 72(12), 3043–3056 (2018)
J.E.B. Randles, A cathode ray polarography. Part. II—the current-voltage curves. Trans. Faraday Soc. 44, 327–338 (1948)
A. Ševčík, Oscillographic polarography with periodical triangular voltage. Collect. Czech. Chem. Commun. 13, 349–377 (1948)
T. Al-Gahouari et al., Resolution improvement for anodic stripping signals of lead and detached indium from reduced graphene oxide/indium tin oxide (rGO/ITO) electrode using bromide ion. Appl. Phys. A 127(5), 326 (2021)
P.M. Lee et al., Reduced graphene oxide decorated with tin nanoparticles through electrodeposition for simultaneous determination of trace heavy metals. Electrochim. Acta 174, 207–214 (2015)
N. Yusoff, Chapter 7 - graphene-polymer modified electrochemical sensors, in Graphene-based electrochemical sensors for biomolecules. ed. by A. Pandikumar, P. Rameshkumar (Elsevier, Amsterdam, 2019), pp.155–186
S.E. Bahinting et al., Bismuth film-coated gold ultramicroelectrode array for simultaneous quantification of Pb(II) and Cd(II) by square wave anodic stripping voltammetry. Sensors 21, 1811 (2021)
Chapter 5 Preconcentration of environmental samples, in Comprehensive Analytical Chemistry (Elsevier: Amsterdam, 1999), p. 185–215. https://doi.org/10.1016/S0166-526X(99)80007-5
J. Wang et al., Hierarchical sulfur-doped graphene foam embedded with Sn nanoparticles for superior lithium storage in LiFSI-based electrolyte. ACS Appl. Mater. Interfaces (2019). https://doi.org/10.1021/acsami.9b10613
M. Wang et al., High-performance tin phosphide/carbon composite anode for lithium-ion batteries. Dalton Trans. (2020). https://doi.org/10.1039/D0DT03139A
Y. Sayato, WHO guidelines for drinking-water quality. Eisei Kagaku 35(5), 307–312 (1989)
M. Jin et al., Review of the distribution and detection methods of heavy metals in the environment. Anal. Methods 12(48), 5747–5766 (2020)
Y. Wei et al., SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II): an interesting favorable mutual interference. J. Phys. Chem. C 116(1), 1034–1041 (2012)
M. Ramalingam, V.K. Ponnusamy, S.N. Sangilimuthu, A nanocomposite consisting of porous graphitic carbon nitride nanosheets and oxidized multiwalled carbon nanotubes for simultaneous stripping voltammetric determination of cadmium(II), mercury(II), lead(II) and zinc(II). Microchim. Acta 186(2), 69 (2019)
S.J. Prabakar, C. Sakthivel, S.S. Narayanan, Hg(II) immobilized MWCNT graphite electrode for the anodic stripping voltammetric determination of lead and cadmium. Talanta 85(1), 290–297 (2011)
G. Zhao, H. Wang, G. Liu, Sensitive determination of trace Cd(ii) and Pb(ii) in soil by an improved stripping voltammetry method using two different in situ plated bismuth-film electrodes based on a novel electrochemical measurement system. RSC Adv. 8(10), 5079–5089 (2018)
G. Tyler, ICP-OES, ICP-MS and AAS Techniques Compared (Technical notes Horiba Group, 2003). https://www.semanticscholar.org/paper/ICP-OES-%2C-ICP-MS-and-AAS-Techniques-Compared-Tyler-Yvon/3a997ca1a20b00e68003ca04937b2035d0d76ad
M.F. Ahmed et al., Investigating the status of cadmium, chromium and lead in the drinking water supply chain to ensure drinking water quality in Malaysia. Water 12(10), 2653 (2020)
Acknowledgements
The authors appreciate the technical support provided by the staff of the School of Materials & Mineral Resources Engineering, Universiti Sains Malaysia. This study was supported by Ministry of Higher Education Malaysia, Fundamental Research Grant Scheme (Grant No. FRGS/1/2020/TK0/USM/01/1), Malaysia Tin Board (304.PBahan. 6050478.L114), and RU Top Down Universiti Sains Malaysia (1001/Pbahan/870049).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramli, N.H., Loo, J.Y., Mohamad Nor, N. et al. Tin nanoparticle-modified electrode for the simultaneous detection of cadmium (Cd) and lead (Pb) ions. J Mater Sci: Mater Electron 35, 73 (2024). https://doi.org/10.1007/s10854-023-11871-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11871-z