Abstract
The nanocomposites of biopolymers and bimetallic oxides are exciting classes of materials. Besides the economic and environmental considerations, these materials became the best candidates for various applications in industry and medicine. In this study, CuWO4 nanoparticles (NP) with high purity were prepared by co-precipitation and loaded into poly(vinyl pyrrolidone)/chitosan (PVP/Cs) films. XRD results showed that CuWO4 has a triclinic phase with an average crystallite size of 43 nm. PVP/Cs is semi-crystalline blend and its crystallinity degraded by CuWO4 incorporation. EDX analysis was used to study the chemical composition of all samples. FE-SEM showed that CuWO4 has particle sizes of 50–150 nm and that the crack-free surface of PVP/Cs became rougher and more porous after loading of CuWO4 NP. FTIR confirmed the presence of the reactive functional group of CuWO4, PVP, and Cs and that the low doping ratio of CuWO4 NP restricted the functional group’s vibrations. The UV–vis–NIR investigation showed that the films have a small absorption index and high transmittance in the range of 68–90%. The direct and indirect band gaps (\({E}_{g}^{dir}\) and \({E}_{g}^{ind}\)) of the blend were found equal to 5.0 and 4.2 eV and can be tuned by CuWO4 content. Similarly, the index of refraction and carrier concentration/electron effective mass ratio (N/m*), the dielectric constant (ε′ = 8.3–24.5), and the dielectric loss depend on the applied frequency, temperature, and CuWO4 filler content. The conductivity (σac) ranges from 1.2 × 10–6 to 9.16 × 10–4 S/m and exhibits the Arrhenius behavior. The optical and dielectric results show that the prepared PNC may suit some energy storage device,s such as supercapacitors, and organic optoelectronic devices, such as light emitting diodes and/or photovoltaic solar cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the development of nanocomposites based on biopolymers mixed with bimetallic oxides has attracted increasing attention. The predominant reason evoked for this trend is the lower cost and ease of polymer nanocomposites (PNC) preparation with properties that make them suitable candidates for potential applications in various fields of industry, such as environment-friendly electronics, food packaging, and medical and pharmaceutical applications [1,2,3,4,5,6].
Among the bimetallic oxides, the copper tungstates (CuWO4) have attracted considerable attention as photoelectrode owing to its high redox, excellent chemical stability of the crystalline CuWO4 in aqueous solutions, excellent catalytic performance, and the moderate band gap (Eg = 2.2–2.4 eV) which permits a theoretical photocurrent density up to 10.7 mA/cm2 [7]. It exhibits a photoresponse with a cut-off wavelength of ~ 540 nm. Besides, the metal-to-metal (Cu2+ → W6+) charge transfer in CuWO4 results in an absorption band at 850 nm. According to Chen et al. [8], the Eg value can be tuned by adjusting the elemental chemical composition CuWO4, where an increase in Cu/W ratio from 0.33 to 2.5 decreases the Eg to 1.88 eV. Moreover, CuWO4 is an n-type semiconductor, and the valence band (VB) and conduction band (CB) positions are positive (+ 2.6–2.7 VRHE and + 0.4 VRHE, respectively) on the normal hydrogen electrode scale, leading to the generation of photo-excited holes with strong oxidation ability [9]. The VB consists of strongly hybridized states of O 2p and Cu 3d, while the CB minimum comprises unoccupied Cu 3d states [7]. These properties make the material a candidate for novel functions in the fields of optics, fluorescence detection [10], photodegradation and water decomposition reactions, and fuel cell reactions. Currently, CuWO4 has gained extensive interest in electrochemical sensors and supercapacitor applications [5, 11].
CuWO4 in the form of nanopowder and films was prepared by several routes; Nong et al. [5] designed an electrochemical Chit-Au/CuWO4@MoS2 immunosensor that showed reproducibility, stability, and good repeatability for detecting cortisol. Lee et al. [7] fabricated CuWO4 films on FTO substrates by the sol–gel method and spin-coating followed by thermal annealing for PEC water splitting. Serwar et al. [9] prepared CuWO4 NP and CuWO4/graphene QDs photocatalysts by co-precipitation and hydrothermal methods, respectively. Sun et al. [10] prepared CuWO4 via the polyacrylamide gel method and obtained the pure triclinic phase after calcination of the xerogel at 900 °C. Du et al. [12] prepared MWO4 (M = Co, Ni, Zn, and Cu) nanosheets on Ni foam by a hydrothermal method for efficient urea oxidation. Jatav et al. [13] fabricated AgI/CuWO4 by a precipitation method for efficient visible-light photocatalysts for ciprofloxacin and RhB degradation.
One of the naturally occurring and abundant cationic polysaccharides is the chitosan (Cs) biopolymer. Cs obtained from chitin by alkaline N-acetylation via a thermochemical reaction. The low cost, high nitrogen content, biodegradability, hydrophilic properties, nontoxicity, reproducibility, good molecular biocompatibility, and antioxidant, antibacterial, and immunomodulatory properties make Cs a promising polymer for various biomedical, biotechnological, and green packaging applications, as well as for artificial skin, bone substitutes, agriculture, and cosmetics. Because of OH and NH2 groups, Cs can exhibit interesting chelating and film-forming properties. The NH2 serves as an electron donor, containing a lone pair electron. It serves the process of complexing and coordinating between the cations of loaded filler into the electrolyte membrane of batteries and acting as a sensor to detect glucose, catechol, and hazardous mercury [4, 14,15,16,17,18]. Moreover, Cs have a semi-crystalline nature and mixing them with another polymer may improve and widen the blend’s multi-functionality. In this context, poly(vinyl pyrrolidone) (PVP) is an amorphous polymer and could improve the film-forming ability. In addition, PVP has a higher resistivity than Cs to ultraviolet rays. Therefore, adding PVP into Cs will maintain or improve the physicochemical properties and expand the Cs utilization to include the electrochemical applications, such as batteries and displays [19, 20].
Few reports on PNC based on CuWO4 filler are found. Thiruppathi et al. [6] prepared CuWO4/PMMA nanocomposite as a photocatalyst for some dyes and antibiotics. To the best of the authors’ knowledge, this is the first attempt to explore the effect of CuWO4 on the physicochemical properties of the PVP/Cs biopolymer blend. This report focuses on preparing CuWO4 by the co-precipitation route and CuWO4/PVP/Cs nanocomposites by solution casting. The morphological, structural, chemical composition, optical, and dielectric properties of the PVP/Cs biopolymer loaded with different filler ratios were investigated. As mentioned above, we aim to enhance and expand the technological and medical applications of PVP/Cs bio-blend.
2 Experimental section
2.1 Preparation of CuWO4 NPs
Sigma-Aldrich provided copper (II) chloride (CuCl2) and sodium tungstate dihydrate (Na2WO4.2H2O) as Cu and W sources. The co-precipitation method generated pure CuWO4 NPs; the Cu and W salts were first dissolved in different beakers containing 400 mL of deionized water. Both of these solutions were mixed and magnetized strongly for 30 min. The CuWO4 precipitate was centrifuged and washed three times with deionized water. Finally, a dark green precipitate of pure CuWO4 NPs was produced by drying the washed CuWO4 sample at 200 °C for two h before grinding it into powder.
2.2 Preparation of PVP/Cs/ CuWO4 nanocomposite
Cs powder (˃ 70% deacetylated) of molecular weight (MW) ~ 1.1 × 105 g/mol., PVP of k = 30, and MW = 4 × 104 g/mol., were used to prepare the PVP/Cs blend. 1.0 g of Cs was dissolved in 75 ml of 2 wt% acetic acid (CH3COOH) using magnetic stirring for 2 h. 0.25 g of PVP in 20 ml distilled water solution was added to this solution, and the stirring continued for one hour at RT. The obtained homogeneous solution was cast into Petri dishes of diameters of ~ 10 cm. For CuWO4/PVP/Cs PNC, the desired mass x (1.0, 3.0, and 5.0%) of CuWO4 was calculated according to the following equation:
where \({W}_{filler}\) is the mass of CuWO4 and “1.25” in the denominator is the total mass of the polymers (PVP + Cs). The calculated \({W}_{filler}\) was dissolved in distilled water using an ultrasonic bath and magnetic stirring and then added to the PVP solution. The films’ composition is summarized in Table 1. The Petri dishes were put on leveled plates and left to dry at 45 °C for ~ 2 days. When removing the films, care was taken to obtain films with uniform thickness for optical and dielectric measurements.
2.3 Characterization
The surface morphology and elemental composition of CuWO4 and CuWO4/PVP/Cs PNC films, the cross-section, and film thickness were studied using field emission-scanning electron microscopy (FE-SEM; Carl ZEISS Sigma 500 VP, coupled with EDS). The X-ray diffraction patterns of CuWO4 and CuWO4/PVP/Cs were recorded using XRD Shimadzu 6000 diffractometer, in the 2θ range of 4°–90°, with a Cu Kα source of wavelength λ = 1.5406 Å. The vibrational modes of the samples were studied using Fourier transform infrared (FTIR) spectroscopy (JASCO, FT/IR-6200) in the wavenumber range 4000–400 cm−1.
The water uptake (WA %), the porosity (P %), and the density (ρ, g/cm3) were investigated using a piece of dimensions (1 cm × 1 cm) from each sample. From the dry (Wd) and wet (Ww) weights of the sample, the ρ (g/cm3), WA (%), and P (%) were measured using the following relations [21,22,23,24,25,26]:
where ρw = 1 g/cm3 and Wo is the initial weight of the sample.
Optical measurements (absorbance, transmittance, and reflectance) were evaluated using a Shimadzu UV-3600 UV–VIS–NIR spectrophotometer at room temperature (RT) and in the wavelength range 200–1600 nm, with an accuracy of ± 0.2 nm.
The dielectric constant (\({\epsilon }^{{\prime }}\)), loss tangent \((\text{tan}\delta )\), and ac conductivity (\({\sigma }_{\text{a}\text{c}})\) were measured in the frequency range 200 Hz–8 MHz and temperature range 293–393 K, using a Hioki model 3532 High Tester LCR (Ueda, Nagano, Japan), with a highly accurate and stable capacitance measurement of the order of 1.0 × 10−4 pF. The following equations were used:
where d denotes the thickness of CuWO4/PVP/Cs film, r represents the radius of CuWO4/PVP/Cs film contact area, εo = 8.854 × 10−12 F/m is the dielectric permittivity of the air, Cp is the capacitance, and ε\({\prime }{\prime }\) is the dielectric loss.
3 Results and discussion
3.1 Structural and morphological properties
The XRD pattern of the co-precipitated CuWO4 is shown in Fig. 1a. In this pattern, the peaks at 2θ = 15.06°, 18.92°, 22.88°, 24.01°, 25.82°, 28.58°, 29.32°, 30.06°, 32.04°, 35.54°, 36.73°, 38.91°, and 55.5° are indexed to the (010), (100), (110), (011), \(\left(\stackrel{-}{1}01\right)\), \(\left(\stackrel{-}{1}\stackrel{-}{1}1\right)\), (111), (020), \(\left(\stackrel{-}{1}11\right)\), (002), (021), \(\left(\stackrel{-}{1}20\right)\), and \(\left(1\stackrel{-}{1}1\right)\), respectively, of CuWO4. Other peaks of fewer intensities and their corresponding to Miller indices are shown in Fig. 1a. This pattern corresponds to the triclinic (an orthic) phase of CuWO4 (SG: P1) and is consistent with the data of JCPDS file 88–0269 [9, 13]. The high intensity and narrow width of the XRD peaks indicate the crystallization quality of the CuWO4 [27]. Considering the three major peaks at 29.32° (111), 28.58° \(\left(\stackrel{-}{1}\stackrel{-}{1}1\right)\), and 32.04° \(\left(\stackrel{-}{1}11\right)\)of relative intensities of 100, 28, and 27% and full-width at half maximum intensity (β) of 0.166, 0.233, and 0.174, respectively, the crystallite size D was evaluated using the well-known Scherrer equation: \(D= \frac{0.9 \text{x} 0.154 \left(nm\right)}{\beta \text{cos} \left(\theta \right)}\), where 0.154 nm is the wavelength (λ) of the used Cu Kα radiation. The calculated D values were in the range of 32.38–49.44 nm and Dav = 43.1 nm. Similarly, Thiruppathi et al. [6] prepared CuWO4 with Dav = 44 nm, using 0.1-M CuCl2 and Na2WO4.2H2O via a hydrothermal process.
The inset of Fig. 1a shows the FE-SEM image of CuWO4 that seems as particles of various sizes in the range of 50–150 nm. This value is bigger than Dav given by XRD. This difference is because SEM gives the particle or grain size, while each grain may contain several crystallites. For the sol–gel prepared Y2O3 NPs the grain size was slightly smaller than 100 nm and Dav was in the range of 18.4–19.5 nm [28]. Figure 1b shows the EDX spectrum of CuWO4 and the mass (%) and atom (%) ratios of O, W, and Cu are listed in the inserted table. The sample is composed of (i) 87.48% oxygen, where the O Kα line appears at ~ 0.5 keV, (ii) 6.83% tungsten, where W Mα, W Lα, and W Lβ emission lines appear at ~ 1.78, 8.4, and 9.7 keV, respectively. (iii) The Cu represents 5.96% where its Lα and Kα lines are at 0.93 and 8.05 keV, respectively. The C Kα line appears at 0.27 keV arising from the carbon grid and the holder of the sample. The O, W, and Cu atoms are uniformly distributed through the whole sample, as indicated by the elemental mapping inserted in Fig. 1b and Fig. S1 (see the supplementary materials file).
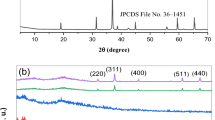
Figure 2 shows the X-ray diffraction patterns of PVP/Cs and CuWO4/PVP/Cs PNC. The pure blend exhibits characteristic peaks at 2θ ≈ 8.5°, 11.2°, 18.1°, and 22.85°. The first three peaks indicate the existence of crystalline or arranged regions in the blend, which could arise due to the intramolecular interaction between the OH and NH2 groups in the blend [29]. However, the wide peak at 2θ = 22.85° belongs to the amorphous phase [30, 31]. Three main notes here: (i) the blend has a semi-crystalline nature. (ii) CuWO4 has been distributed effectively in the amorphous regions of the blend and the XRD instrument is not able to detect this nanofiller. (iii) The intensity of the crystalline peaks decreased, and the wide of the amorphous peak increased with increasing CuWO4 loading. The crystallinity degree \({X}_{C}\text{\%}=\frac{{A}_{c} \text{x} 100}{{A}_{c}+{A}_{a}}\), where \({A}_{c}\) and \({A}_{a}\) are the areas under the crystalline and amorphous peaks, respectively. \({X}_{c}\) of the pure blend is 18.5% decreased to 12.6% at 1.0-wt% CuWO4 loading and marginally decreased till 10.4% with the further increase in CuWO4 ratio 5.0 wt%. This decrease in XC indicates increasing the biodegradability of the blend loaded with CuWO4 [32], and the blend flexibility and chain motion which in turn will improve the conductivity of the material.
Figure 3 shows the EDS spectra, chemical (elemental) composition, and the inset tables of PVP/Cs loaded with 1.0- and 3.0-wt% CuWO4. In the two samples, the main components are C (55.64–56.49 at%), O (33.42–33.60 at%), and N (7.87–8.57%). These ratios are consistent with the chemical structure of both Cs (C56H103N9O39)n and PVP (C6H10NO)n. Although XRD did not detect any peaks for CuWO4, EDX confirmed the existence of Cu (0.5–1.25 at%) and W (1.02–1.65 at%). The Cu appears at 0.93 keV (Lα1), 0.95 keV (Lβ1), 8.05 keV (Kα1), and 8.9 keV (Kα1). The W emissions appear at 1.85 KeV (Mα), 8.4 keV (Lα1), and 9.7 keV (Lβ1). This result confirms the successful incorporation of CuWO4 inside PVP/Cs chains. Moreover, the inserted tables and SEM images confirm the homogeneous distribution and increase of [Cu] and [W] with increasing CuWO4 doping ratio. To shed more light on the surface morphology of pure and CuWO4/PVP/Cs films, FE-SEM images were captured and presented as shown in Fig. 4a–c. PVP/Cs have a smooth, homogeneous, and crack-free surface, Fig. 4a. This means the homogeneity and complete miscibility of PVP and Cs polymers. At higher magnification, a very large number of nanopores exist on the film’s surface. In addition, the cross-sectional investigation emphasizes that these pores do not exist inside the blend. Loading of 3.0- and 5.0-wt% CuWO4 was uniformly distributed into the blend matrix but induced some heterogeneity and the surface became rougher. These fillers encourage the pores to form on the surface and inside the matrix, with uniform distribution, as seen in the insets of Fig. 4b and c. Moreover, the films have a thickness range of 45–57 μm. The induced structural changes could influence other physical properties such as the values of ρ, P %, and WA% of the PNC.
The influences of CuWO4 content on WA %, P %, and ρ are shown in Fig. 5. The WA % of CuWO4/PVP/Cs decreases from 216.9 to 80.66% with increasing CuWO4 nanofillers loading from 0.0 to 5.0 wt%. This reduction in WA% may be due to the hydrophobic characteristics of the added metal oxide (CuWO4) [21, 22]. The strong adhesion of CuWO4 nanofiller at the PVP/Cs interfaces leads to increasing the porosity from 105.86, for the pure blend, to 129.71% for the blend loaded with 5.0-wt% CuWO4 [23]. This is consistent with the observation of FE-SEM, Fig. 4. Additionally, incorporation of this dense metal oxide (ρCuWO4 = 7.48 g/cm3) result in increasing the ρ of CuWO4/PVP/Cs PNC from 0.507 to 1.633 g/cm3. Similar results were reported in [24,25,26].
3.2 FTIR spectra of CuWO 4 and PVP/Cs PNC
FTIR is a powerful and interesting tool to clarify the vibration modes of the material functional groups and the interaction/complexation of the blend chains with the added nanofiller. FTIR spectra were recorded at RT and are shown in Fig. 6. The spectrum of CuWO4 displays several absorption peaks. This material can adsorb the water vapor from the surrounding [27], where the bands at ~ 3430 and 1640 cm–1 are ascribed to the –OH stretching and H–O–H bending vibrations. The band at 610 cm–1 could be assigned to the Cu–O stretching in the triclinic CuWO4. The bands at 729 and 812 are assigned to the stretching modes of O–W–O/ W–O–W groups. In addition, the bands at 472 and 903 cm–1 are assigned to the stretching vibration of WO4 tetrahedra [6, 10, 33]. This result indicates the high purity of our co-precipitated CuWO4.
The spectra of CuWO4/PVP/Cs PNC contain a wide and broadband in the range of 3000–3600 cm–1, which can be divided into two bands, as shown in Fig. 6; the first centered at 3360 cm–1 can be assigned to the asymmetrical stretching of N–H group in the PVP/Cs blend [34], and the second is centered at 3265 cm–1 arising from the OH stretching vibration overlapping with the symmetric stretching o N–H through the carbohydrate ring in the CS [1, 4]. Similarly, the two bands at 2935 and 2875 cm–1 arise from the C–H symmetric and asymmetric stretching vibrations in the CH2 groups [35]. The absorption band at 1650 cm–1 is attributed to the –C=O stretching of amide I in Cs. The relatively broad and strong band at 1547 cm−1 can be assigned to the symmetric deformation of \(-N{H}_{3}^{+}\) (resulting from the ionization of NH2 groups by adding acetic acid during the dissolving Cs) [36]. In addition, the stretching vibration of –C=N of amide-III in Cs appears at 1397 cm–1 [35]. The small absorption band at 1290 cm–1 is owing to the frequencies of (CH + OH) combination [37]. The three absorption bands at 1150 cm–1, 1068 cm–1, and 1025 cm–1 can be ascribed to the asymmetric stretching vibration of C–O–C and the stretching of C–O in the C–OH groups [18]. The small band at 647 cm−1 may be owing to twisting vibrations in the blend [38]. All films have similar spectra and no obvious shift in the peak position due to CuWO4 incorporation. However, it is seen that the width and intensity of the peaks decreased after loading 1.0-wt% CuWO4 but increased with increasing the ratio of the filler to 3.0 and 5.0 wt%. This result indicates that 1.0 wt% is the more effective ratio in restricting the vibrations of PVP/Cs blend functional groups.
3.3 UV–vis study and optical constants
Investigating the optical properties of semi-crystalline materials and determining their optical constants are important for elucidating the electronic and band structures for optical communication and device fabrications. Figure 7a and b shows the absorption index (\(k=\frac{\alpha \lambda }{4\pi })\) and transmittance spectra (T%) of the CuWO4/PVP/Cs PNT, where \(\alpha =\frac{2.303 Abs.}{d}\) and d is the film thickness. The films show low k values (k ≤ 2.0 × 10−3), Fig. 7a. The PVP/Cs show two weak absorption peaks at 278 and 310 nm, assigned to π→π* and n→π* transitions, respectively, that are arising due to the existence of unsaturated bonds [39]. The intensity of these two peaks is significantly improved after doping with 1.0-wt% CuWO4 and then decreased and left-shifted with increasing loading ratio of CuWO4 to 3.0 and 5.0 wt%. This results from the induced changes in XC% of the PVP/Cs after mixing with CuWO4 [40], as shown in XRD results. For comparison purposes, the value of T% at 600 nm of all samples is listed in Table 2. At λ ≥ 600 nm, the PVP/Cs show a motivating T% in the range of 85–90%, Fig. 7b. The PNC films have T = 57% at λ = 600 nm. In the studied λ range, 1.0-wt% CuWO4/PVP/Cs film exhibits a maximum T of 78% decreases to 68% with increasing the ratio of the filler to 5.0 wt%. With decreasing the λ toward the UV region, the T% of the films decreases sharply, and the absorption edge shifts to higher λ especially at 1.0-wt% doping, indicating a narrowing of the optical band gap of the blend. The T% values of the films emphasize the homogeneity and CuWO4/PVP/Cs composites and their convenience for optical devices.
Using Tauc’s relations: \((\alpha h\upsilon {)}^{1/m}=A(h\nu -{E}_{g})\) [4], where \(h\nu\) is the incident photon energy (\(\sim\frac{1242 \left(\text{e}\text{V}\right)}{\lambda \left(\text{n}\text{m}\right)})\), C is a constant, m = \(\frac{1}{2}\) or 2 for allowed direct and indirect transitions, respectively, and the direct (\({E}_{g}^{dir}\)) and indirect (\({E}_{g}^{ind})\) optical band gap can be determined. Figure 8a and b shows the \((\alpha h\upsilon {)}^{2}\) vs. \(h\nu\) and \({\left(\alpha h\upsilon \right)}^{1/2}\) vs. hυ. Extrapolating the linear parts of these plots to α = 0, gives the \({E}_{g}^{dir}\) and \({E}_{g}^{ind}\) values which are listed in Table 2. PVP/Cs blend has \({E}_{g}^{dir}\) and \({E}_{g}^{ind}\) of 5.0 and 4.2 eV, respectively, significantly reduced to 4.4 and 3.1 eV after loading 1.0-wt% CuWO4. However, increasing the content of the filler to 5.0 wt% widens the \({E}_{g}^{dir}\) and \({E}_{g}^{ind}\) of PNC films to 4.8 and 3.8 eV, respectively, but still lower than the values of the pure blend. In the previous work, the incorporation of 2.0-wt% hematite nanorods narrowed the \({E}_{g}^{dir}\) of PVP/Cs from 5.4 eV to 4.05 eV [1]. Abdelghany et al. [20] reported \({E}_{g}^{dir}\) and \({E}_{g}^{ind}\) of about 4.77 and 4.14 eV for a blend composed of PVP (80%)/Cs (20%) which widened to 4.9 and 4.47 eV, respectively, after doping with 0.002 g of ZnS NP. CuWO4 filler (Eg = 2.2–2.4 eV) forms a semiconducting network inside the blend and create defects, charge transfer complexes, as well as create localized states in the forbidden region between the conduction and valence bands of the blend. This results in the observed shrinkage in Eg. However, increasing the filler content beyond 1.0 wt% may reduce the efficiency of this network due to the agglomeration of the nano-sized filler owing to its huge surface energy.
Evaluating the index of refraction (n) of the materials is essential for optical device applications. The recorded reflectance (R%) and (\(n=\frac{1+\sqrt{R}}{1-\sqrt{R}}\) [41]) of the films are shown in Fig. 9a and b. Both R% and n decrease with λ in the UV region. In the visible region, the added CuWO4 filler made the R% and n behavior take a bell shaped. Insertion of CuWO4 inside PVP/Cs improves its reflectivity due to the scattering effect of the dispersed particles. CuWO4/PVP/Cs PNC has a high n and may be utilized in some top-end advanced optoelectronic equipment, such as light-emitting diodes, waveguides, and anti-reflective coatings. Considering the dispersion relation: \({n}^{2}={\epsilon }_{l}-\left(\frac{{e}^{2}}{{\pi c}^{2}}\right)\left(\frac{N}{{m}^{*}}\right){\lambda }^{2}\) [1], where εl, e, c, and \(\frac{N}{{m}^{*}}\) are the dielectric constant of lattice, electron charge, speed of light, and the ratio of carrier concentration to the effective mass of the electron, respectively. The \(\frac{N}{{m}^{*}}\) value can be easily from the plot of n2 vs. λ2 as shown in Fig. 9c. The slope of straight lines gives the values of \(\frac{N}{{m}^{*}}\left(\frac{{e}^{2}}{{\pi c}^{2}}\right)\) as listed in Table 2. The value of \(\frac{N}{{m}^{*}}\left(\frac{{e}^{2}}{{\pi c}^{2}}\right)\) increased from 1.96 × 10–7 to 1.37 × 10–6nm−2 after loading 1.0-wt% CuWO4 into the blend but slightly reduced to 1.06 × 10–6 and 6.52 × 10–7 nm−2 with increasing the content of the filler to 3.0 and 5.0 wt%, respectively. The observed change in \(\frac{N}{{m}^{*}}\left(\frac{{e}^{2}}{{\pi c}^{2}}\right)\) is consistent with the variation in \({E}_{g}^{dir}\) and \({E}_{g}^{ind}\) of PNC films, where increasing/decreasing carrier concentration results in decreasing/increasing the Eg values. Moreover, these results indicate that the optical properties of the PVP/Cs blend can be tuned by the ratio of CuWO4 nanofiller. These enhancements in the optical properties make these compositions suitable for optoelectronic devices, such as organic light-emitting diodes and photovoltaic cells [42].
3.4 Dielectric constant and loss of CuWO4/PVP/Cs
Improving the dielectric property of a material by increasing the number of charges it can store is essential for device applications, such as supercapacitors and batteries. Figure 10a–d and Fig. S2 exhibit the variation of PVP/Cs dielectric constant (ɛ′), before and after loading of 1.0–5.0-wt% CuWO4, at temperatures (293–393 K) and frequencies (f = 2.0 kHz–4.0 MHz). Increasing the applied f results in a continuous decrease in the ɛ′ values, Fig. S2. At low f, the accumulated charge carriers yield a high ɛ′ and tend to build up a space-charge layer at the interface between the PVP/Cs film and the electrode, and any charge has the time required to change the direction according to the change in f-direction. However, increasing f is combined with a short periodic time in which the dipoles fail to reorient fast enough. Therefore, the dipolar polarization diminishes and may disappear and hence ɛ′ values decrease [43, 44]. The temperature dependence is somewhat different. The curves (ɛ′ vs. T) can be divided into two regions, in the first one, the ε′ increases with increasing the temperature that supplies thermal energy enough to free localized dipoles which in turn align themselves in the f-direction. In the second region, the high temperatures increase the specific volume of the blend, hence the dipole concentration (dipole number/unit volume) decreases resulting in the observed decrease in ε′. The separation between the two regions is called the relaxation peak or the α-process, which shifts to the right with increasing the applied f. This α-process is assigned to the micro-Brownian motion along the PVP/Cs main chains found in its amorphous regions.
Two additional notes on the behavior of ε′: (i) the dispersion of the curves at a lower temperature is higher than that at higher temperatures. This means f has the decisive effect at low temperatures, and this effect reduces at a higher temperature, after the α-process. (ii) At low temperatures, the PVP/Cs blend has ε′ in the range of 8.3–17.8 that significantly increased to 9.8–24.5 after loading 1.0-wt% CuWO4, where the heterogeneity created inside the blend and the contribution of the interfacial polarization increase ε′. However, a further increase in the added CuWO4 ratio decreases the ε′ range to 9.0–19, but still higher than that of the pure blend. The higher filler content may not be distributed uniformly and induce structural changes that act as trapping centers for the charges.
The dependence of the dielectric loss (ε″) with f at different temperatures is given in Fig. 11a–d and Fig. S3. At the low f (≤ 40 kHz), the ε″ is small and decreases with increasing temperatures up to a certain limit (the gained thermal energy is small and cannot affect the motion of the PVP/Cs chains) then increases with temperature (the temperature above a certain limit improves the motion of the chains and rise ɛ″). At higher f (≥ 0.2 MHz), ɛ″ increases with increasing temperature till a certain limit then decreases with further increase in the temperature, where the free charge carriers, the polar groups in the blend, and the motion of the chain become fail to in phase with the oscillating f. Moreover, the observed relaxation peaks in the PVP/Cs and CuWO4/PVP/Cs spectra shifted to higher f with increasing temperature, Fig. 11, or shifted to higher temperatures with increasing f, Fig. S3. The higher values of ε″ at f ˃ 1.0 MHz is a result of increasing the molecular vibrations in the crystalline regions in the PVP/Cs blend [45]. In the middle region of the studied frequencies (3.0 kHz ≤ f ≤ 1.0 MHz), the maximum values of ɛ″ for PVP/Cs and 5.0-wt% CuWO4/PVP/Cs the blend is around 2.75 regardless of the temperature. Increases slightly to 3.0 and ~ 4 for 3.0- and 1.0-wt% CuWO4/PVP/Cs, respectively. This may indicate the fact that the crystalline regions in this semi-crystalline blend dissolve effectively in their amorphous regions with increasing temperature [46]. Incorporation of CuWO4 into PVP/Cs blend improved ε′ significantly maintaining similar ε″ values. These composites, therefore, can be further improved to be suitable for batteries and supercapacitors (energy storage devices), and the electrostriction systems utilized in the artificial muscles [47]. Moreover, 1.0-wt% CuWO4/PVP/Cs has the higher ε′ and therefore the higher dielectric displacement (\(D={\epsilon }_{o}{\epsilon }^{{\prime }}E)\) and high energy density (\({U}_{e}=\int EdD)\), in the presence of the applied electric field E [48].
The conductivity (σac) of the dielectric materials (, ω = 2πf is the angular frequency) is related to the temperature by the Arrhenius equation: , where kB is the Boltzmann constant, σo is a pre-exponential factor, Ea is the activation energy, and T is the temperature (K). The conductivity of pure and 1.0-wt% CuWO4-doped PVP/Cs and the log (σac) vs. 1000/T are shown in Fig. 12. The σac of PVP/Cs is in the order of 1.2 × 10–6–7.3 × 10–4 S/m and this high σac may be due to the polycationic characteristic of the blend. The NPs of CuWO4 semiconductor may form 3D connected networks inside the blend where the σac range of 1.0-wt% CuWO4/PVP/Cs increased to 4.95 × 10–6–9.16 × 10–4 S/m. The σac curves could be divided into two regions showing an Arrhenius behavior; in the region I, the conductivity improvement is due to the available thermal activation of the PVP/Cs polymeric chains. The decreasing trend with increasing T, in Region II, is related to the decrease in the carrier concentration and the structural changes in the blend with heating.
4 Conclusion
CuWO4 NP and CuWO4/PVP/Cs PNC were successfully prepared with co-precipitation and solution casting techniques. XRD and EDX showed the formation of CuWO4 in the triclinic crystal structure with Dav = 43.1 nm, where the sample was found to composed of 87.48 at.% O, 6.83 at.% W, and 5.96 at.% Cu with a uniform distribution. PVP/Cs is a semi-crystalline blend and \({X}_{C}\) of the blend decreased from 18.5 to 10.4% after doping, where the EDX analysis confirmed the presence of both Cu and W in the blend. FE-SEM showed that the grain size of CuWO4 is in the range of 50–150 nm. PVP/Cs has a crack-free surface and the incorporation of CuWO4 NP induced some heterogeneity, roughness, and pores formation and increased both the density and porosity of the PVP/Cs. FTIR confirmed the high purity of CuWO4 and the existence of all the reactive functional groups of the polymers, which were more restricted at 1.0-wt% CuWO4 loading. The films exhibited small k values (≤ 2.0 × 10−3) and the transmittance of the blend, which decreased from 85–90–68% at 5.0-wt% CuWO4 doping ratio. Direct and indirect band gaps were decreased from \({E}_{g}^{dir}\) and \({E}_{g}^{ind}\) from 5.0 and 4.2 eV to 4.4 and 3.1 eV at 1.0 wt%, but then increased to 4.8 and 3.8 eV, respectively. Both the index of refraction and \(\frac{N}{{m}^{*}}\) increased from 1.79 and 1.96 × 10–7 nm–2 to 2.29 and 1.37 × 10–6 nm–2 after doping with 1.0-wt% CuWO4 and then decreased with increasing filler content. The ε′ of PVP/Cs is in the range of 8.3–17.8, increased to 9.8–24.5 at 1.0-wt% filler content. The maximum values of ɛ″ for PVP/Cs and CuWO4/PVP/Cs is small (2.75–4.0). The σac increased from 1.2 × 10–6–7.3 × 10–4 S/m to 4.95 × 10–6– 9.16 × 10–4 S/m at 1.0-wt% filler content. In summary, the structural, optical, and dielectric properties can be controlled by CuWO4 NP content. Therefore, these composites can be improved to be suitable for some energy storage devices such as supercapacitors as well as some optoelectronic applications, such as light-emitting diodes and photovoltaic solar cells.
Data availability
Data will be made available on reasonable request.
References
A.M. El Sayed, A.D.M. Mohamad, Synthesis, structural, thermal, optical and dielectric properties of chitosan biopolymer; influence of PVP and α-Fe2O3 nanorods. J. Polym. Res. 25, 175 (2018). https://doi.org/10.1007/s10965-018-1571-x
P.H. Dinh, T.-D. Pham, D.Q. Trung, D.V. Thuan, N.T.D. Cam, N.T. Hanh, H.V. Ha, N.H.A. Thu, H.T. Trang, N.L.M. Tri, CuWO4 decorated by polypyrrole (PPy) protector/sensitizer for novel photocatalytic and stable water splitting for hydrogen generation. Int. J. Hydrogen Energy. 45(41), 21442–21449 (2020). https://doi.org/10.1016/j.ijhydene.2020.05.229
J. Yesuraj, O. Padmaraj, S.A. Suthanthiraraj, Synthesis, characterization, and improvement of Supercapacitor Properties of NiMoO4 nanocrystals with polyaniline. J. Inorg. Organ. Polym. Mater. 30, 310–321 (2020). https://doi.org/10.1007/s10904-019-01189-x
A.M. El Sayed, S. El-Gamal, Synthesis, optical, and electrical properties of starch/chitosan/NaTiO3 bio-nanocomposites modified with ErCl3. Phys. Scr. 97, 015805 (2022). https://doi.org/10.1088/1402-4896/ac40da
C. Nong, B. Yang, X. Li, S. Feng, H. Cui, An ultrasensitive electrochemical immunosensor based on in-situ growth of CuWO4 nanoparticles on MoS2 and chitosan-gold nanoparticles for cortisol detection. Microchem J. 179, 107434 (2022). https://doi.org/10.1016/j.microc.2022.107434
M. Thiruppathi, K. Saravanakumar, M. Arunpandian, C. Ramalingan, E.R. Nagarajan, A novel CuWO4-PMMA nanocomposite thin film as trouble-free and handpicking recoverable photocatalyst. https://doi.org/10.1080/03067319.2021.1951259
J.U. Lee, J.H. Kim, K. Kang, Y.S. Shin, J.Y. Kim, J.H. Kim, J.S. Lee, Bulk and surface modified polycrystalline CuWO4 films for photoelectrochemical water oxidation. Renew. Energy. 203, 779–787 (2023). https://doi.org/10.1016/j.renene.2022.12.129
L. Chen, S. Shet, H. Tang, K.-S. Ahn, H. Wang, Y. Yan, J. Turner, M. Al-Jassim, Amorphous copper tungsten oxide with tunable band gaps. J. Appl. Phys. 108, 043502 (2010). https://doi.org/10.1063/1.3475714
A. Sarwar, A. Razzaq, M. Zafar, I. Idrees, F. Rehman, W.Y. Kim, Copper tungstate (CuWO4)/graphene quantum dots (GQDs) composite photocatalyst for enhanced degradation of phenol under visible light irradiation. Res. Phys. 45, 106253 (2023). https://doi.org/10.1016/j.rinp.2023.106253
G. Sun, Q. Gao, S. Tang, X. Chen, H. Liu, H. Gao, X. Zhao, A. Wang, X. Yu, S. Wang, Facile synthesis, Optical and Photoluminescence Properties of Copper Tungstate Phosphors with strong Near-Infrared Photoabsorption. Russ J. Phys. Chem. A 96(6), 1348–1355 (2022). https://doi.org/10.1134/S0036024422060097
A. Hrubantova, R. Hippler, H. Wulff, M. Cada, O. Gedeon, P. Jiricek, J. Houdkova, J. Olejnicek, N. Nepomniashchaia, C.A. Helm, Z. Hubicka, Copper tungsten oxide (CuxWOy) thin films for optical and photoelectrochemical applications deposited by reactive high power impulse magnetron co-sputtering. J. Appl. Phys. 132, 215301 (2022). https://doi.org/10.1063/5.0123075
X. Du, Z. Dai, Y. Wang, X. Han, X. Zhang, Facile synthesis of MWO4 (M = co, Ni, Zn and Cu) nanoarrays for efficient urea oxidation. Int. J. Hydrogen Energy. 47, 8875–8882 (2022). https://doi.org/10.1016/j.ijhydene.2021.12.244
N. Jatav, J. Kuntail, D. Khan, A.K. De, I. Sinha, AgI/CuWO4 Z-scheme photocatalyst for the degradation of organic pollutants: experimental and molecular dynamics studies. J. Colloid Interf Sci. 599, 717–729 (2021). https://doi.org/10.1016/j.jcis.2021.04.120
A.H. Bashal, M. Khalafalla, T.A. Abdel-Basset, Dielectric Properties and AC Conductivity of Chitosan-La2O3 Nanocomposite. Arab. J. Sci. Eng. 46, 5859–5864 (2020). https://doi.org/10.1007/s13369-020-04958-w
V. Bertolino, G. Cavallaro, S. Milioto, G. Lazzara, Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 245, 116502 (2020). https://doi.org/10.1016/j.carbpol.2020.116502
G. Cavallaro, S. Micciulla, L. Chiappisi, G. Lazzara, Chitosan-based smart hybrid materials: a physico-chemical perspective. J. Mater. Chem. B 9(3), 594–611 (2021). https://doi.org/10.1039/D0TB01865A
R.B. Suneetha, P. Selvi, C. Vedhi, Synthesis, structural and electrochemical characterization of Zn doped iron oxide/grapheneoxide/chitosan nanocomposite for supercapacitor application. Vacuum. 164, 396–404 (2019). https://doi.org/10.1016/j.vacuum.2019.03.051
M.I.A. Maksoud, M. Bekhit, D.M. El-Sherif, A.R. Sofy, M.R. Sofy, "Gamma radiation-induced synthesis of a novel chitosan/silver/Mn-Mg ferrite nanocomposite and its impact on cadmium accumulation and translocation in brassica plant growth." Int. J. Biol. Macromol. 194, 306–316 (2022). https://doi.org/10.1016/j.ijbiomac.2021.11.197
A. Sionkowska, M. Wisniewski, J. Skopinska, S. Vicini, E. Marsano, The influence of UV irradiation on the mechanical properties of chitosan/poly(vinyl pyrrolidone) blends. Polym. Degrad. Stab. 88, 261–267 (2005). https://doi.org/10.1016/j.polymdegradstab.2004.08.018
A.M. Abdelghany, M.S. Meikhail, A.H. Oraby, M.A. Aboelwafa, Experimental and DFT studies on the structural and optical properties of chitosan/polyvinyl pyrrolidone/ZnS nanocomposites. Polym. Bull., (2023) in press. DOI:https://doi.org/10.1007/s00289-023-04700-0
A.N. Anyakora, O.K. Abubakre, E. Mudiare, M.A.T. Suleiman, Effect of fibre loading and treatment on porosity and water absorption correlated with tensile behaviour of oil palm empty fruit bunch fibre reinforced composites. Adv. Mater. Res. 6(4), 329 (2017). https://doi.org/10.12989/amr.2017.6.4.329
S.A. Baskakov, Y.V. Baskakova, E.V. Dvoretskaya, S.S. Krasnikova, V.A. Lesnichaya, Y.M. Shulga, G.L. Gutsev, Mechanical and water absorption Properties of Waterborne Polyurethane/Graphene oxide composites. Materials. 16(1), 178 (2022). https://doi.org/10.3390/ma16010178
A. Paydayesh, L. Heleil, A.S. Dadkhah, Preparation and application of poly (hydroxyl ethyl methacrylate) nanocomposite hydrogels containing iron oxide nanoparticles as wound dressing. Polym. Polym. Compos. 30, 1–10 (2022). https://doi.org/10.1177/09673911211063106
V.R. Pereira, A.M. Isloor, U.K. Bhat, I.A.F. smail, A. Obaid, H.K. Fun, Preparation and performance studies of polysulfone-sulfated nano-titania (S-TiO2) nanofiltration membranes for dye removal. RSC Adv. 5(66), 53874–53885 (2015). https://doi.org/10.1039/C5RA07994B
P.K. Panda, J.M. Yang, Y.H. Chang, Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 257, 117633 (2021). https://doi.org/10.1016/j.carbpol.2021.117633
A. Karul, K.T. Tan, C.C. White, D.L. Hunston, S.T. Marshall, B. Akgun, S.K. Satija, C.L. Soles, B.D. Vogt, Impact of polymer modulus/chain mobility on water accumulation at polymer/metal oxide interfaces. Polymer. 50(14), 3234–3239 (2009). https://doi.org/10.1016/j.polymer.2009.04.064
H. Halakoei, M. Ghalkhani, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, An efficient electrochemical sensor based on CeVO4-CuWO4 nanocomposite for methyldopa. Mater. Res. Express. 8, 085001 (2021). https://doi.org/10.1088/2053-1591/ac16f1
G. Mohammed, S. El-Gamal, A.M. El Sayed, S. Saber, Synthesis, structural, optical and electrical characterization of Y2O3/poly(ethylene glycol)–poly(vinyl chloride) based nanocomposite solid polymer electrolytes. Polym. Int. 72, 342–355 (2023). https://doi.org/10.1002/pi.6472
S. Sakkara, D. Nataraj, K. Venkatesh, N. Reddy, Influence of Alkali Treatment on the Physicochemical and Mechanical Properties of Starch Chitosan Films. Starch. 71, 1800084 (2019). https://doi.org/10.1002/star.201800084
G.B. Patel, N.L. Singh, F. Singh, Modification of chitosan-based biod egradable polymer by irradiation with MeV ions for electrolyte applications. Mater. Sci. Eng. B 225, 150–159 (2017). https://doi.org/10.1016/j.mseb.2017.08.023
S. Wang, L. Shen, W. Zhang, Y. Tong, Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules. 6, 3067–3072 (2005). https://doi.org/10.1021/bm050378v
E.S. Costa-Júnior, E.F. Barbosa-Stancioli, A.A.P. Mansur, W.L. Vasconcelos, H.S. Mansur, Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 76, 472–481 (2009). https://doi.org/10.1016/j.carbpol.2008.11.015
B. Kavitha, R. Karthiga, Synthesis and characterization of CuWO4 as nano-adsorbent for removal of Nile blue and its antimicrobial studies. J. Mater. Environ. Sci. 11(1), 57–68 (2020)
H. Dweik, W. Sultan, M. Sowwan, S. Makharza, Analysis characterization and some Properties of Polyacrylamide Copper Complexes. “Int J. Polym. Mater. Po. 57(3), 228–244 (2008). https://doi.org/10.1080/00914030701413280
H. Mittal, A.A. Alili, P.P. Morajkar, S.M. Alhassan, GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose – A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biolog Macromolec. 167, 1248–1261 (2021). https://doi.org/10.1016/j.ijbiomac.2020.11.079
J. Ahmed, M. Mulla, Y.A. Arfat, L.A. Thai, Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocolloid. 71, 141–148 (2017). https://doi.org/10.1016/j.foodhyd.2017.05.013
G. Mohammed, A.M. El Sayed, W.M. Morsi, Spectroscopic, thermal, and electrical properties of MgO/ polyvinylpyrrolidone/ polyvinyl alcohol nanocomposites. J. Phys. Chem. Solids. 115, 238–247 (2018). https://doi.org/10.1016/j.jpcs.2017.12.050
C. Song, H. Yu, M. Zhang, Y. Yang, G. Zhang, Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biolog Macromol. 60, 347–354 (2013). https://doi.org/10.1016/j.ijbiomac.2013.05.039
A.M. El Sayed, G. Khabiri, Spectroscopic, Optical and Dielectric Investigation of (mg, Cu, Ni, or cd) acetates’ influence on Carboxymethyl Cellulose Sodium Salt/Polyvinylpyrrolidone Polymer Electrolyte Films. J. Electron. Mater. 49(4), 2381–2392 (2020). https://doi.org/10.1007/s11664-020-07953-x
M. Morsi, A. Rajeh, A. Menazea, Nanosecond laser-irradiation assisted the improvement of structural, optical and thermal properties of polyvinyl pyrrolidone/carboxymethyl cellulose blend filled with gold nanoparticles. J. Mater. Sci. : Mater. Electron. 30, 2693–2705 (2019). https://doi.org/10.1007/s10854-018-0545-4
T.I. Alanazi, A.M. El, Sayed, Characterization of Mg–Pb–O systems, and MgPbO–thermoplastic blend: nanocomposites for photonic and microelectronic devices. J. Phys. Chem. Solids. 178, 111346 (2023). https://doi.org/10.1016/j.jpcs.2023.111346
T.-P. Nguyen, Polymer-based nanocomposites for organic optoelectronic devices. A review. Surf. Coat. Technol. 206, 742–752 (2011). https://doi.org/10.1016/j.surfcoat.2011.07.010
A.M. El Sayed, W.M. Morsi, Dielectric relaxation and Optical Properties of polyvinyl Chloride/Lead Monoxide Nanocomposites. Polym. Compos. 34, 2031–2039 (2013). https://doi.org/10.1002/pc.22611
T. Fahmy, H. Elhendawi, W.B. Elsharkawy, F.M. Reicha, AC conductivity and dielectric relaxation of chitosan/poly(vinyl alcohol) biopolymer polyblend. Bull. Mater. Sci. 43, 243 (2020). https://doi.org/10.1007/s12034-020-02207-2
H.I. Elsaeedy, H.E. Ali, H. Algarni, I.S. Yahia, Nonlinear behavior of the current–voltage characteristics for erbiumdoped PVA polymeric composite films. Appl. Phys. A 125, 79 (2019). https://doi.org/10.1007/s00339-018-2375-x
D.C. Bharati, P. Rawat, A.L. Saroj, Structural, thermal, and ion dynamics studies of PVA-CS-NaI-based biopolymer electrolyte films. J. Solid State Electrochem. 25, 1727–1741 (2021). https://doi.org/10.1007/s10008-021-04946-6
M.K. Mishra, S. Moharana, B. Behera, R.N. Mahaling, Surface functionalization of BiFeO3: a pathway for the enhancement of dielectric and electrical properties of poly(methyl methacrylate)–BiFeO3 composite films. Front. Mater. Sci. 11(1), 82–91 (2017). https://doi.org/10.1007/s11706-017-0364-1
A.M. El, Sayed, Aspects of structural, optical properties, and relaxation in (BiFeO3 or NaTiO3)–PMMA: hybrid films for dielectric applications. J. Phys. Chem. Solids. 148, 109767 (2021). https://doi.org/10.1016/j.jpcs.2020.109767
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). None.
Author information
Authors and Affiliations
Contributions
AMElS contributed to conceptualization; methodology; data curation; writing of the original draft; and editing of the manuscript. MIAAM contributed to conceptualization; methodology; data curation; investigation; writing of the original draft; and writing, reviewing, & editing of the manuscript. SMK contributed to data curation and writing of the original draft. ASA contributed to data curation; writing of the original draft; and writing, reviewing, & editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Sayed, A.M., Abdel Maksoud, M.I.A., Kassem, S.M. et al. Synthesis, structural, optical, and dielectric properties of CuWO4/PVP/Cs bio-nanocomposites for some industrial applications. J Mater Sci: Mater Electron 34, 1713 (2023). https://doi.org/10.1007/s10854-023-11118-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11118-x