Abstract
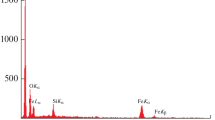
Venlafaxine (VEN) is one of the antidepressants belonging to the general family of the selective norepinephrine and serotonin reuptake inhibitors. However, an overdose of VEN might cause the symptoms of serotonin toxicity, depression, cardiac conduction abnormalities, or seizure. In the current work, a simple, cost-efficient, and sensitive electrochemical sensor for determination of venlafaxine was described. Firstly, Fe3O4@SiO2/MWCNT (FSMW) nanocomposite was synthesized and after characteristics assesses its, has been utilized for modification of screen-printed electrode (FSMW/SPE) as working electrode for electrochemical determination of venlafaxine. Differential pulse voltammetry (DPV), chronoamperometry (CHA), and cyclic voltammetry (CV) have been also employed for electrochemical detection of venlafaxine and characterization of the modified electrode. The electron transfer coefficient (α = 0.3) and diffusion coefficient (D = 2.9 × 10− 6 cm2 s−1) of venlafaxine oxidation at the surface of FSMW/SPE were determined using electrochemical approaches. Venlafaxine’s reduction peak current was greatly increased by the modified electrode while its overpotential was decreased. The FSMW/SPE showed a linear current response for oxidation of venlafaxine between 0.5 and 200.0 µM with a 0.3 µM limit of detection (LOD). Additionally, the modified electrode has been effectively used to identify venlafaxine’s existence in actual samples.





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Any raw data files should be needed in another format they are available from the corresponding author upon reasonable request.
References
E. Eslami, F. Farjami, Voltammetric determination of venlafaxine by using multiwalled carbon nanotube- ionic liquid composite electrode. J. Appl. Chem. Res. 12, 42–52 (2018)
B. Maddah, M. Cheraghveisi, M. Najafi, Developing a modified electrode based on La3+/Co3O4 nanocubes and its usage to electrochemical detection of venlafaxine. Int. J. Environ. Anal. Chem. 100, 121–133 (2020)
T. Madrakian, R. Haryani, M. Ahmadi, A. Afkhami, A sensitive electrochemical sensor for rapid and selective determination of venlafaxine in biological fluids using carbon paste electrode modified with molecularly imprinted polymer-coated magnetite nanoparticles. J. Iran Chem. Soc. 13, 243–251 (2016)
C.J. Whittington, T. Kendall, P. Fonagy, D. Cottrell, A. Cotgrove, E. Boddington, Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 363, 1341–1345 (2004)
T. Madrakian, R. Haryani, M. Ahmadi, A. Afkhami, Spectrofluorometric determination of venlafaxine in biological samples after selective extraction on the superparamagnetic surface molecularly imprinted nanoparticles. Anal. Methods. 7, 428–435 (2015)
M. Matoga, F. Pehourcq, K. Titier, F. Dumora, C. Jarry, Rapid high-performance liquid chromatographic measurement of venlafaxine and O-desmethylvenlafaxine in human plasma. Application to management of acute intoxications. J. Chromatogr. B 760, 213–218 (2001)
R. Mandrioli, L. Mercolini, R. Cesta, S. Fanali, M. Amore, M.A. Raggi, Analysis of the second generation antidepressant venlafaxine and its main active metabolite O-desmethylvenlafaxine in human plasma by HPLC with spectrofluorimetric detection. J. Chromatogr. B 856, 88–94 (2007)
O. Mastrogianni, G. Theodoridis, K. Spagou, D. Violante, T. Henriques, A. Pouliopoulos, K. Psaroulis, H. Tsoukali, N. Raikos, Determination of venlafaxine in post-mortem whole blood by HS-SPME and GC-NPD. Forensic Sci. Int. 215, 105–109 (2012)
J. Bhatt, A. Jangid, G. Venkatesh, G. Subbaiah, S. Singh, Liquid chromatography-tandem mass spectrometry (LC-MS-MS) method for simultaneous determination of venlafaxine and its active metabolite O-desmethyl venlafaxine in human plasma. J. Chromatogr. B 829, 75–81 (2005)
S. Rudaz, C. Stella, A.E. Balant-Gorgia, S. Fanali, J.L. Veuthey, Simultaneous stereoselective analysis of venlafaxine and O-desmethylvenlafaxine enantiomers in clinical samples by capillary electrophoresis using charged cyclodextrins. J. Pharm. Biomed. Anal. 23, 107–115 (2000)
M. Kingbäck, M. Josefsson, L. Karlsson, J. Ahlner, F. Bengtsson, F.C. Kugelberg, B. Carlsson, Stereoselective determination of venlafaxine and its three demethylated metabolites in human plasma and whole blood by liquid chromatography with electrospray tandem mass spectrometric detection and solid phase extraction. J. Pharm. Biomed. Anal. 53, 583–590 (2010)
H. Beitollahi, S. Jahani, S. Tajik, M.R. Ganjali, F. Faridbod, T. Alizadeh, Voltammetric determination of venlafaxine as an antidepressant drug employing Gd2O3 nanoparticles graphite screen printed electrode. J. Rare Earth. 37, 322–328 (2019)
B.J. Sanghavi, A.K. Srivastava, Adsorptive stripping differential pulse voltammetric determination of venlafaxine and desvenlafaxine employing Nafion–carbon nanotube. Electrochim. Acta. 56, 4188–4196 (2011)
S. Ariavand, M. Ebrahimi, E. Foladi, Design and construction of a novel and an efficient potentiometric sensor for determination of sodium ion in urban water samples. Chem. Methodol. 6, 886–904 (2022)
H. Karimi-Maleh, Y. Liu, Z. Li, R. Darabi, Y. Orooji, C. Karaman, F. Karimi, M. Baghayeri, J. Rouhi, L. Fu, Calf thymus ds-DNA intercalation with pendimethalin herbicide at the surface of ZIF-8/Co/rGO/C3N4/ds-DNA/SPCE; a bio-sensing approach for pendimethalin quantification confirmed by molecular docking study. Chemosphere 332, 138815 (2023)
J. Mohanraj, D. Durgalakshmi, R.A. Rakkesh, S. Balakumar, S. Rajendran, H. Karimi-Maleh, Facile synthesis of paper based graphene electrodes for point of care devices: a double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 566, 463–472 (2020)
Z. Zhang, H. Karimi-Maleh, In situ synthesis of label-free electrochemical aptasensor-based sandwich-like AuNPs/PPy/Ti3C2Tx for ultrasensitive detection of lead ions as hazardous pollutants in environmental fluids. Chemosphere. 324, 138302 (2023)
H. Peyman, H. Roshanfekr, A. Babakhanian, H. Jafari, PVC membrane electrode modified by lawson as synthetic derivative ionophore for determination of cadmium in alloy and wastewater. Chem. Methodol. 5, 446–453 (2021)
S.Z. Mohammadi, H. Beitollahi, H. Allahabadi, T. Rohani, Disposable electrochemical sensor based on modified screen printed electrode for sensitive cabergoline quantification. J. Electroanal. Chem. 847, 113223 (2019)
J.A. Buledi, N. Mahar, A. Mallah, A.R. Solangi, I.M. Palabiyik, N. Qambrani, F. Karimi, Y. Vasseghian, Karimi-Maleh, Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: a potential method for environmental remediation. Food Chem. Toxicol. 161, 112843 (2022)
Z. Mehdizadeh, S. Shahidi, A. Ghorbani-HasanSaraei, M. Limooei, M. Bijad, Monitoring of amaranth in drinking samples using voltammetric amplified electroanalytical sensor. Chem. Methodol. 6, 246–252 (2022)
S. Cheraghi, M.A. Taher, H. Karimi-Maleh, F. Karimi, M. Shabani-Nooshabadi, M. Alizadeh, A. Al-Othman, N. Erk, P.K.Y. Raman, C. Karaman, Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere. 287, 132187 (2022)
S.Z. Mohammadi, H. Beitollahi, M. Jasemi, A. Akbari, Nanomolar determination of methyldopa in the presence of large amounts of hydrochlorothiazide using a carbon paste electrode modified with graphene oxide nanosheets and 3-(4’-Amino-3’-hydroxy-biphenyl-4-yl)- acrylic acid. Electroanalysis. 27, 2421–2430 (2015)
H. Karimi-Maleh, C.T. Fakude, N. Mabuba, G.M. Peleyeju, O.A. Arotiba, The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 554, 603–610 (2019)
M. Bijad, H. Karimi-Maleh, M. Farsi, S.A. Shahidi, An electrochemical-amplified-platform based on the nanostructure voltammetric sensor for the determination of carmoisine in the presence of tartrazine in dried fruit and soft drink samples. J. Food Meas. Charact. 12, 634–640 (2018)
H. Roshanfekr, A simple specific dopamine aptasensor based on partially reduced graphene oxide–Au NPs composite. Prog. Chem. Biochem. Res. 6, 79–88 (2023)
Z. Zhang, H. Karimi-Maleh, Label-free electrochemical aptasensor based on gold nanoparticles/titanium carbide MXene for lead detection with its reduction peak as index signal. Adv. Compos. Hybrid. Mater. 6, 68 (2023)
S.Z. Mohammadi, H. Beitollahi, M. Mousavi, Determination of hydroxylamine using a carbon paste electrode modified with graphene oxide nano sheets. Russ. J. Electrochem. 53, 374–379 (2017)
A. Hosseini Fakhrabad, R. Sanavi Khoshnood, M.R. Abedi, M. Ebrahimi, Fabrication a composite carbon paste electrodes (CPEs) modified with multi-wall carbon nano-tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 3, 627–634 (2021)
S.Z. Mohammadi, H. Beitollahi, Z. Dehghan, R. Hosseinzadeh, Electrochemical determination of ascorbic acid, uric acid and folic acid using carbon paste electrode modified with novel synthesized ferrocene derivative and core–shell magnetic nanoparticles in aqueous media. Appl. Organomet. Chem. 32, 4551 (2018)
S.Z. Mohammadi, F. Mousazadeh, S. Tajik, Simultaneous determination of doxorubicin and dasatinib by using screen-printed electrode/Ni – fe layered double hydroxide. Ind. Eng. Chem. Res 62, 4646–4654 (2023)
H. Karimi-Maleh, R. Darabi, F. Karimi, C. Karaman, S.A. Shahidi, N. Zare, M. Baghayeri, L. Fu, S. Rostamnia, J. Rouhi, State-of-art advances on removal, degradation and electrochemical monitoring of 4-aminophenol pollutants in real samples: a review. Environ. Res 222, 115338 (2023)
S.Z. Mohammadi, S. Tajik, H. Beitollahi, Screen printed carbon electrode modified with magnetic core shell manganese ferrite nanoparticles for electrochemical detection of amlodipine. J. Serb Chem. Soc. 84, 1005–1016 (2019)
Y. Zou, X. Zhou, L. Xie, H. Tang, F. Yan, Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 10, 939510 (2022)
Y. Zeng, M.B. Camarada, X. Lu, K. Tang, W. Li, D. Qiu, L. Bai, Detection and electrocatalytic mechanism of zearalenone using nanohybrid sensor based on copper-based metal-organic framework/magnetic Fe3O4-graphene oxide modified electrode. Food Chem. 370, 131024 (2022)
S.Z. Mohammadi, H. Beitollahi, M. Kaykhaii, N. Mohammadizadeh, S. Tajik, R. Hosseinzadeh, Simultaneous determination of droxidopa and carbidopa by carbon paste electrode functionalized with NiFe2O4 nanoparticle and 2-(4-ferrocenyl-[1,2,3]triazol-1-yl)-1-(naphthalen-2-yl) ethenone. Measurement. 155, 107522 (2020)
Y. Shao, Y. Zhu, R. Zheng, P. Wang, Z. Zhao, J. An, Highly sensitive and selective surface molecularly imprinted polymer electrochemical sensor prepared by au and MXene modified glassy carbon electrode for efficient detection of tetrabromobisphenol A in water. Adv. Compos. Hybrid. Mater. 5, 3104–3116 (2022)
S.Z. Mohammadi, H. Beitollahi, M. Hassanzadeh, Voltammetric determination of tryptophan using a carbon paste electrode modified with magnesium core shell nanocomposite and ionic liquids. Anal. Bioanal Chem. Res 5, 55–65 (2018)
S.Z. Mohammadi, H. Beitollahi, S. Tajik, Nonenzymatic coated screen-printed electrode for electrochemical determination of acetylcholine. Micro Nano Syst. Lett. 6, 1–7 (2018)
F. Hasanpour, M. Taei, M. Fouladgar, M. Salehi, Au nano dendrites/composition optimized nd-dopped cobalt oxide as an efficient electrocatalyst for ethanol oxidation. J. Appl. Organomet. Chem. 2, 188–196 (2022)
H. Peyman, Design and fabrication of modified DNA-Gp nano-biocomposite electrode for industrial dye measurement and optical confirmation. Prog. Chem. Biochem. Res. 5, 391–405 (2022)
L. Alwan, E. Al Samarrai, M. Mahmood, Q. Ali, O.A. Samarrai, Estimation and development of some biophysical characteristics of the drug Favipiravir used in the treatment of corona-virus using green chemistry technology. Eurasian Chem. Commun. 4, 835–851 (2022)
S. Janitabar Darzi, H. Bastami, Au decorated mesoporous TiO2 as a high performance photocatalyst towards crystal violet dye. Adv. J. Chem. A 5, 22–30 (2022)
I. Sheikhshoaie, A. Rezazadeh, S. Ramezanpour, Removal of pb (II) from aqueous solution by gel combustion of a new nano sized Co3O4/ZnO composite. Asian J. Nanosci. Mater. 5, 336–345 (2022)
V. Tallapaneni, L. Mude, D. Pamu, V.V.S.R. Karri, Formulation, characterization and in vitro evaluation of dual-drug loaded biomimetic chitosan-collagen hybrid nanocomposite scaffolds. J. Med. Chem. Sci. 5, 1059–1074 (2022)
R. Shete, P. Fernandes, B. Borhade, A. Pawar, M. Sonawane, N. Warude, Review of cobalt oxide nanoparticles: green synthesis, biomedical applications, and toxicity studies. J. Chem. Rev. 4, 331 (2022)
H. Shayegan, V. Safarifard, H. Taherkhani, M.A. Rezvani, Efficient removal of cobalt(II) ion from aqueous solution using amide-functionalized metal-organic framework. J. Appl. Organomet. Chem. 2, 109–118 (2022)
F. Nareetsile, J.T. Matshwele, S. Odisitse, Metallo-drugs as promising antibacterial agents and their modes of action. J. Med. Chem. Sci. 5, 1109–1131 (2022)
M. Sharma, S. Yadav, M. Srivastava, N. Ganesh, S. Srivastava, Promising anti-inflammatory bio-efficacy of saponin loaded silver nanoparticles prepared from the plant Madhuca longifolia. Asian J. Nanosci. Mater. 5, 313–326 (2022)
T.S.K. Naik, A.V. Kesavan, B.K. Swamy, S. Singh, A.G. Anil, V. Madhavi, P.C. Ramamurthy, Low cost, trouble-free disposable pencil graphite electrode sensor for the simultaneous detection of hydroquinone and catechol. Mater. Chem. Phys. 278, 125663 (2022)
F.E. Ettadili, S. Aghris, F. Laghrib, A. Farahi, M. Bakasse, S. Lahrich, E. Mhammedi, M. A, Electrochemical detection of ornidazole in commercial milk and water samples using an electrode based on green synthesis of silver nanoparticles using cellulose separated from Phoenix dactylifera seed. Int. J. Biol. Macromol. 242, 124995 (2023)
F. Wang, D. Zhao, W. Li, H. Zhang, B. Li, T. Hu, L. Fan, Rod-shaped units based cobalt (II) organic framework as an efficient electrochemical sensor for uric acid detection in serum. Microchem. J. 185, 108154 (2023)
S.Z. Mohammadi, H. Beitollahi, M. Kaykhaii, N. Mohammadizadeh, A. Novel Electrochemical, Sensor based on graphene oxide nanosheets and ionic liquid binder for differential pulse voltammetric determination of droxidopa in pharmaceutical and urine samples. Rus J. Electrochem. 55, 1229–1236 (2019)
X.J. Huang, A.M. O’Mahony, R.G. Compton, Microelectrode arrays for electrochemistry: approaches to fabrication. Small. 5, 776–788 (2009)
J.P. Metters, R.O. Kadara, C.E. Banks, New directions in screen printed electroanalytical sensors: an overview of recent developments. Analyst. 136, 1067–1076 (2011)
A. Gevaerd, C.E. Banks, M.F. Bergamini, Marcolino-Junior, graphene quantum dots modified screen‐printed electrodes as electroanalytical sensing platform for diethylstilbestrol. Electroanalysis. 31, 838–843 (2019)
F. Garkani Nejad, H. Beitollahi, I. Sheikhshoaie, A UiO-66-NH2 MOF/PAMAM dendrimer nanocomposite for electrochemical detection of tramadol in the presence of acetaminophen in pharmaceutical formulations. Biosensors. 13, 514 (2023)
V.C. Valsalakumar, A.S. Joseph, J. Piyus, S. Vasudevan, Polyaniline–graphene oxide composites decorated with ZrO2 nanoparticles for use in screen-printed electrodes for real-time l-tyrosine sensing. ACS Appl. Nano Mater. 6, 8382–8395 (2023)
S. Moru, V. Sunil Kumar, S. Kummari, K. Yugender Goud, A disposable screen printed electrodes with hexagonal ni (OH)2 nanoplates embedded chitosan layer for the detection of depression biomarker. Micromachines. 14, 146 (2023)
M. Bartolomé, M.L. Soriano, M.J. Villaseñor, Ã. Ríos, γ-Cyclodextrin-graphene quantum dots-chitosan modified screen-printed electrode for sensing of fluoroquinolones. Microchim. Acta. 190, 60 (2023)
H. Beitollahi, F. Ebadinejad, F. Shojaie, M. Torkzadeh, Mahani, Magnetic core–shell Fe3O4@SiO2/MWCNT nanocomposite modified carbon paste electrode for amplified electrochemical sensing of amlodipine and hydrochlorothiazide. Anal. Methods. 8, 6185–6193 (2016)
Z. Lu, J. Dai, X. Song, G. Wang, W. Yang, Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloids Surf. A 317, 450–456 (2008)
H. Duan, X. Wang, Y. Wang, J. Li, C. Luo, Bioreceptor multi-walled carbon nanotubes@Fe3O4@SiO2–surface molecular imprinted polymer in an ultrasensitive chemiluminescent biosensor for bovine hemoglobin. RSC Adv. 5, 88492 (2015)
V.W.O. Wanjeri, S. Gbashi, J.C. Ngila, P. Njobeh, M.A. Mamo, P.G. Ndungu, Chemical Vapour Deposition of MWCNT on Silica Coated Fe3O4 and Use of Response Surface Methodology for Optimizing the Extraction of Organophosphorus Pesticides from Water. Int. J. Anal. Chem. 2019, 4564709 (2019)
S.Z. Mohammadi, H. Beitollahi, H. Fadaeian, Voltammetric determination of isoproterenol using a graphene oxide nano sheets paste electrode. J. Anal. Chem. 73, 705–712 (2018)
A.J. Bard, L.R. Faulkner, Electrochemical methods: fundamentals and applications, 2nd edn. (Wiley, New York, 2001), pp.100–137
L. Ding, L. Li, W. You, Z.N. Gao, T.L. Yang, Electrocatalytic oxidation of venlafaxine at a multiwall carbon nanotubes-ionic liquid gel modified glassy carbon electrode and its electrochemical determination. Croatica Chem. Acta. 88, 81–87 (2015)
M.A. Khalilzadeh, S. Tajik, H. Beitollahi, R.A. Venditti, Green synthesis of magnetic nanocomposite with iron oxide deposited on cellulose nanocrystals with copper (Fe3O4@CNC/Cu): investigation of catalytic activity for the development of a venlafaxine electrochemical sensor. Ind. Eng. Chem. Res. 59, 4219–4228 (2020)
P. Mohammadzadeh Jahani, H. Akbari Javar, H. Mahmoudi-Moghaddam, A new electrochemical sensor based on Europium-doped NiO nanocomposite for detection of venlafaxine. Measurement. 173, 108616 (2021)
S. Morais, C.P.M.C.A. Ryckaert, C. Delerue-Matos, Adsorptive stripping voltammetric determination of venlafaxine in urine with a mercury film microelectrode. Anal. Lett. 36, 2515–2526 (2003)
Acknowledgements
The Bam University of Medical Sciences, Bam, Iran, provided financial assistance for this project (no. 98000075), which the authors gratefully thank.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by FM and YN. The first draft of the manuscript was written by FM and YN. The Revise & Editing was done by SZM. The verification and data curation were done by ST. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadi, S.Z., Tajik, S., Mousazadeh, F. et al. Constructing a simple and sensitive electrochemical sensor for the determination of venlafaxine based on Fe3O4@SiO2/MWCNT nanocomposite-modified screen-printed electrode. J Mater Sci: Mater Electron 34, 1641 (2023). https://doi.org/10.1007/s10854-023-11030-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11030-4